CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Auxiliary Activity Family 13"
Harry Brumer (talk | contribs) (Fixed Davies & Sinnott ref.) |
|||
| Line 1: | Line 1: | ||
| − | |||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
{{UnderConstruction}} | {{UnderConstruction}} | ||
| − | * [[Author]]: ^^^Leila | + | * [[Author]]: ^^^Glyn Hemsworth^^^ & ^^^Leila LoLeggio^^^ |
* [[Responsible Curator]]: ^^^Gideon Davies^^^ | * [[Responsible Curator]]: ^^^Gideon Davies^^^ | ||
---- | ---- | ||
| Line 13: | Line 12: | ||
|- | |- | ||
|'''Clan''' | |'''Clan''' | ||
| − | |Structurally related to [[AA9]] & [[ | + | |Structurally related to [[AA9]], [[AA10]] & [[AA11]] |
|- | |- | ||
|'''Mechanism''' | |'''Mechanism''' | ||
| Line 19: | Line 18: | ||
|- | |- | ||
|'''Active site residues''' | |'''Active site residues''' | ||
| − | |mononuclear copper ion | + | |mononuclear copper ion coordinated by the “histidine brace” |
|- | |- | ||
|{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | |{{Hl2}} colspan="2" align="center" |'''CAZy DB link''' | ||
| Line 29: | Line 28: | ||
| + | == Substrate specificities == | ||
| + | The AA13 family represents the fourth family of Lytic Polysaccharide Monooxygenases (LPMOs) that has been identified. Found in fungi, the first member of this family to be identified and characterised was isolated from <i>Neurospora crassa</i> by Vu et al <cite>Vu2014</cite>. While searching the <i>Neurospora crassa</i> genome for new putative LPMO sequences, they identified a sequence that appeared to code for an LPMO with a C-terminal [[CBM20]] domain but lacked significant overall sequence similarity to other [[AA9]], [[AA10]] or [[AA11]] LPMOs. Not long after Lo Leggio et al <cite>LoLeggio2015</cite> also identified and characterised AA13 family members from <i>Aspergilllus nidulans</i> and <i>Aspergillus oryzae</i> using a similar approach. | ||
| + | All AA13s that have been biochemically characterised to date are active on starch <cite>Vu2014 LoLeggio2015</cite>. The expression of these enzymes has also been shown to be heavily unregulated during growth of <i>A. nidulans</i> on this substrate <cite>Nekiunaite2016a</cite>. As for other LPMOs, AA13s utilise copper and an electron donor to oxidatively introduce chain breaks into the α-1,4-linked glucose polymers that form starch <cite>Vu2014 LoLeggio2015</cite>. AA13s specifically attack at the C1 position of the sugar ring forming oligosaccharide products with lactones at the reducing end which are then additionally hydrated to form aldonic acid terminated maltodextrins <cite>LoLeggio2015</cite>. The C1 specificity suggests that these LPMOs should be able to oxidatively attack both the α-1,4- and α-1,6-linkages found in amylopectin <cite>Vu2015</cite>. Interestingly, AA13s are currently the only LPMOs characterised to date that act on a glucan polymer formed from anything other than β-1,4-linkages <cite>Vu2016</cite>. | ||
| − | == | + | == Kinetics and Mechanism == |
| − | + | All LPMOs ([[AA9]], [[AA10]], [[AA11]], & AA13) are copper dependent monooxygenases and there is considerable work ongoing in trying to elucidate their detailed reaction mechanism <cite>Walton2017</cite>. Density Functional Theory (DFT) calculations have been performed using [[AA9]] structures as a starting point leading to the currently favoured mechanism being a Cu(II)-oxyl based rebound mechanism <cite>Kim2014 Bertini2017</cite>. Starch, being an α-1,4-linked substrate, poses a different challenge for AA13s to be able to oxidatively attack at the C1 carbon. Whether there is a significant difference in mechanism required in order to oxidise starch is currently unclear. | |
| + | |||
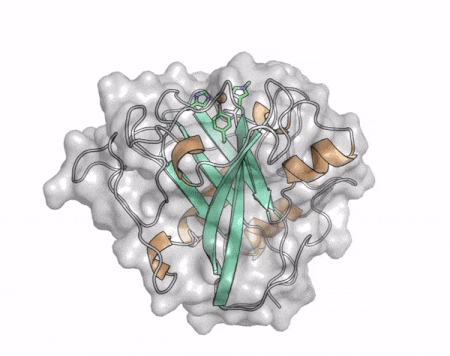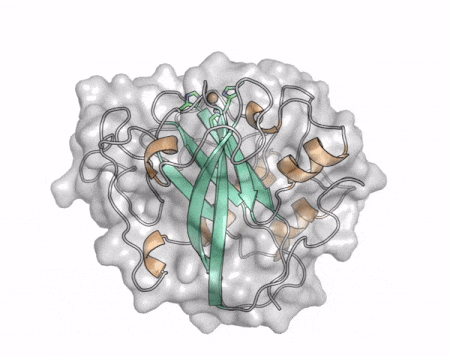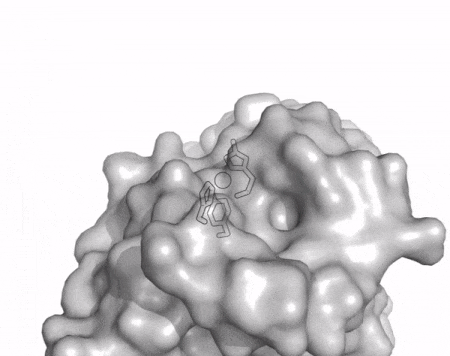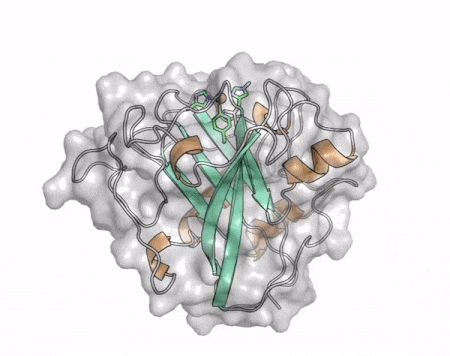
| + | [[File:AA13_Cazypedia_Small.gif|thumb|right|450x356px|'''Figure 1. Structure of AA13 from <i>Aspergillus oryzae</i> (PDB ID [{{PDBlink}}4opb 4OPB] <cite>LoLeggio2015</cite>).''' In the above animation the structure is shown initially in cartoon representation coloured by secondary structure and the transparent surface shown in grey. The active site is shown at the top of the structure with the copper ion shown as a sphere and surrounding active site residues shown as sticks with green carbon atoms. As the animation proceeds the surface surrounding the active site is shown to illustrate the presence of a groove that passes over the active site. This groove is thought to accommodate the helical structure of an α-1,4-linked amylopectin substrate.]] | ||
| − | + | Performing kinetic measurements on LPMOs has been notoriously difficult. Several key aspects of AA13 biochemistry have been observed however. The <i>A. nidulans</i> AA13 showed significant synergy in starch degradation assays in combination with a β-amylase, boosting the production of maltose by this enzyme >100-fold, representing one of the most significant boosting activities observed so far for any LPMO <cite>LoLeggio2015</cite>. Also highlighted in this study was the importance of the reducing agent used to activate the enzyme with cysteine providing greater activity than ascorbate which is typically used to activate LPMOs. Vu et al <cite>Vu2014</cite> also showed that cellobiose dehydrogenase (CDH composed of [[AA3]] and [[AA8]] domains) also represents a good activating partner for AA13s as has been observed for [[AA9]]s. The importance of the [[CBM20]] module for AA13 activity has also been highlighted. The <i>A. oryzae</i> AA13, which naturally lacks a CBM, did not appear to be active in assays on starch while the [[CBM20]] appended A.nidulans enzyme showed clear activity <cite>LoLeggio2015</cite>. Indeed the presence of the [[CBM20]] domain has been demonstrated to confer typical starch and β-cyclodextrin binding properties to AA13s and is suggested to mediate most of the substrate binding properties of this family of enzymes <cite>Nekiunaite2017b</cite>. | |
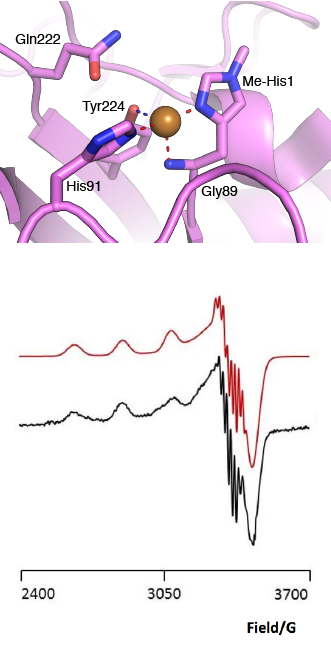
| − | + | [[File:AA13_Active_Site.png|thumb|right|331x654px|'''Figure 2. The active site architecture observed for the AA13 from <i>A. oryzae</i> (PDB ID [{{PDBlink}}4opb 4OPB] <cite>LoLeggio2015</cite>).''' '''Top:''' the active site residues surrounding the copper ion are shown. The Cu is shown as a sphere and is in the Cu(I) state due to photo reduction from the X-ray beam. '''Bottom:''' EPR spectrum observed for the AA13 from <i>A. oryzae</i> revealing super hyperfine coupling. The raw data are shown in black with the simulated data in red.]] | |
| − | == | + | == Three-dimensional structures == |
| − | + | The core fold of the AA13 structure is similar to all other LPMOs ([[AA9]], [[AA10]], & [[AA11]]) representing a β-sandwich immunoglobulin like fold <cite>LoLeggio2015</cite>. There are some significant structural differences compared to the other families however. Firstly, the AA13 structure reveals additional helical secondary structure elements that are not found in other LPMOs (Figure 1). The most prominent difference however is surrounding the copper active site. Where in other LPMOs, the copper histidine brace is found at the centre of a flat or slightly concave surface ([[AA9]], [[AA10]], & [[AA11]]), a distinct groove can be observed passing over the active site of AA13 (Figure 1). This is an adaptation that is thought to be necessary to accommodate the helical structure of an amylopectin substrate. | |
== Catalytic Residues == | == Catalytic Residues == | ||
| − | + | The “histidine brace” motif is used to bind the active site copper in AA13 <cite>LoLeggio2015</cite>, as it is for all other LPMOs studied to date ([[AA9]], [[AA10]] & [[AA11]]) <cite>Vaaje-Kolstad2017</cite>. This motif sees the amino group and imidazole side chain of the N-terminal histidine, together with the imidazole group of a second histidine, directly coordinate the copper ion in a T-shaped geometry. For AA13s, one axial position is occupied by a tyrosine on one side of the copper ion, with a loop containing a glycine approaching near to the copper on the other side <cite>LoLeggio2015</cite>. In [[AA10]]s and [[AA11]]s alanine in this position has been suggested as a possible important factor in distorting the arrangement of water molecules around the active site which may have mechanistic consequences in these families <cite>Hemworth2013 Hemsworth2014</cite>. In addition, the accessibility of this axial position to solvent has been implicated in mediating the regiospecificity of [[AA10]]s and [[AA9]]s, with secondary sphere resides around the copper becoming increasingly studied as well <cite>Forsberg2014 Borisova2015 Span2017 Forsberg2017</cite>. An interesting feature of the electron paramagnetic spectrum observed for the <i>A. nidulans</i> and <i>A. oryzae</i> AA13s was the presence of superhyperfine coupling in the spectrum <cite>LoLeggio2015</cite>. This was inidicative of a potentially more ordered active site arrangement around the copper which may be the result of structural changes that are required in order to oxidise starch. | |
| − | |||
| − | |||
| − | |||
== Family Firsts == | == Family Firsts == | ||
| − | ;First | + | ;First family member identified: AA13 from <i>A. oryzae</i> <cite>LoLeggio2015</cite>. |
| − | ;First | + | ;First demonstration of oxidative cleavage: The <i>N. crassa</i> AA13 was shown to produce oxidised malto-oligosaccharides in the presence of oxygen and reducing agents <cite>Vu2014</cite>. |
| − | + | ;First 3-D structure: AA13 from ''A. oryzae'' with Cu+ [{{PDBlink}}4opb 4OPB] <cite>LoLeggio2015</cite> | |
| − | ;First 3-D structure: | ||
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | # | + | #Vu2014 pmid=25201969 |
| − | # | + | #LoLeggio2015 pmid=25608804 |
| + | #Nekiunaite2016a Nekiunaite, L., Arntzen, M.Ø., Svensson, B., Vaaje-Kolstad, G., Abou Hachem, M. (2016) Lytic polysaccharide monooxygenases and other oxidative enzymes are abundantly secreted by <i>Aspergillus nidulans</i> grown on different starches. Biotechnology for Biofuels. 9, 187 [http://dx.doi.org/10.1186/s13068-016-0604-0] | ||
| + | #Vu2016 pmid=27170366 | ||
| + | #Walton2017 pmid=27094791 | ||
| + | #Bertini2017 pmid=29232119 | ||
| + | #Kim2014 pmid=24344312 | ||
| + | #Nekiunaite2017b pmid=27397613 | ||
| + | #Vaaje-Kolstad2017 pmid=28086105 | ||
| + | #Forsberg2014 pmid=24912171 | ||
| + | #Borisova2015 pmid=26178376 | ||
| + | #Span2017 pmid=28257189 | ||
| + | #Forsberg2017 pmid=29222333 | ||
| + | |||
</biblio> | </biblio> | ||
[[Category:Auxiliary Activity Families|AA13]] | [[Category:Auxiliary Activity Families|AA13]] | ||
Revision as of 14:52, 4 January 2018
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Glyn Hemsworth^^^ & ^^^Leila LoLeggio^^^
- Responsible Curator: ^^^Gideon Davies^^^
| Auxiliary Activity Family 13 | |
| Clan | Structurally related to AA9, AA10 & AA11 |
| Mechanism | lytic oxidase |
| Active site residues | mononuclear copper ion coordinated by the “histidine brace” |
| CAZy DB link | |
| https://www.cazy.org/AA13.html | |
Substrate specificities
The AA13 family represents the fourth family of Lytic Polysaccharide Monooxygenases (LPMOs) that has been identified. Found in fungi, the first member of this family to be identified and characterised was isolated from Neurospora crassa by Vu et al [1]. While searching the Neurospora crassa genome for new putative LPMO sequences, they identified a sequence that appeared to code for an LPMO with a C-terminal CBM20 domain but lacked significant overall sequence similarity to other AA9, AA10 or AA11 LPMOs. Not long after Lo Leggio et al [2] also identified and characterised AA13 family members from Aspergilllus nidulans and Aspergillus oryzae using a similar approach.
All AA13s that have been biochemically characterised to date are active on starch [1, 2]. The expression of these enzymes has also been shown to be heavily unregulated during growth of A. nidulans on this substrate [3]. As for other LPMOs, AA13s utilise copper and an electron donor to oxidatively introduce chain breaks into the α-1,4-linked glucose polymers that form starch [1, 2]. AA13s specifically attack at the C1 position of the sugar ring forming oligosaccharide products with lactones at the reducing end which are then additionally hydrated to form aldonic acid terminated maltodextrins [2]. The C1 specificity suggests that these LPMOs should be able to oxidatively attack both the α-1,4- and α-1,6-linkages found in amylopectin [4]. Interestingly, AA13s are currently the only LPMOs characterised to date that act on a glucan polymer formed from anything other than β-1,4-linkages [5].
Kinetics and Mechanism
All LPMOs (AA9, AA10, AA11, & AA13) are copper dependent monooxygenases and there is considerable work ongoing in trying to elucidate their detailed reaction mechanism [6]. Density Functional Theory (DFT) calculations have been performed using AA9 structures as a starting point leading to the currently favoured mechanism being a Cu(II)-oxyl based rebound mechanism [7, 8]. Starch, being an α-1,4-linked substrate, poses a different challenge for AA13s to be able to oxidatively attack at the C1 carbon. Whether there is a significant difference in mechanism required in order to oxidise starch is currently unclear.
Performing kinetic measurements on LPMOs has been notoriously difficult. Several key aspects of AA13 biochemistry have been observed however. The A. nidulans AA13 showed significant synergy in starch degradation assays in combination with a β-amylase, boosting the production of maltose by this enzyme >100-fold, representing one of the most significant boosting activities observed so far for any LPMO [2]. Also highlighted in this study was the importance of the reducing agent used to activate the enzyme with cysteine providing greater activity than ascorbate which is typically used to activate LPMOs. Vu et al [1] also showed that cellobiose dehydrogenase (CDH composed of AA3 and AA8 domains) also represents a good activating partner for AA13s as has been observed for AA9s. The importance of the CBM20 module for AA13 activity has also been highlighted. The A. oryzae AA13, which naturally lacks a CBM, did not appear to be active in assays on starch while the CBM20 appended A.nidulans enzyme showed clear activity [2]. Indeed the presence of the CBM20 domain has been demonstrated to confer typical starch and β-cyclodextrin binding properties to AA13s and is suggested to mediate most of the substrate binding properties of this family of enzymes [9].
Three-dimensional structures
The core fold of the AA13 structure is similar to all other LPMOs (AA9, AA10, & AA11) representing a β-sandwich immunoglobulin like fold [2]. There are some significant structural differences compared to the other families however. Firstly, the AA13 structure reveals additional helical secondary structure elements that are not found in other LPMOs (Figure 1). The most prominent difference however is surrounding the copper active site. Where in other LPMOs, the copper histidine brace is found at the centre of a flat or slightly concave surface (AA9, AA10, & AA11), a distinct groove can be observed passing over the active site of AA13 (Figure 1). This is an adaptation that is thought to be necessary to accommodate the helical structure of an amylopectin substrate.
Catalytic Residues
The “histidine brace” motif is used to bind the active site copper in AA13 [2], as it is for all other LPMOs studied to date (AA9, AA10 & AA11) [10]. This motif sees the amino group and imidazole side chain of the N-terminal histidine, together with the imidazole group of a second histidine, directly coordinate the copper ion in a T-shaped geometry. For AA13s, one axial position is occupied by a tyrosine on one side of the copper ion, with a loop containing a glycine approaching near to the copper on the other side [2]. In AA10s and AA11s alanine in this position has been suggested as a possible important factor in distorting the arrangement of water molecules around the active site which may have mechanistic consequences in these families [11, 12]. In addition, the accessibility of this axial position to solvent has been implicated in mediating the regiospecificity of AA10s and AA9s, with secondary sphere resides around the copper becoming increasingly studied as well [13, 14, 15, 16]. An interesting feature of the electron paramagnetic spectrum observed for the A. nidulans and A. oryzae AA13s was the presence of superhyperfine coupling in the spectrum [2]. This was inidicative of a potentially more ordered active site arrangement around the copper which may be the result of structural changes that are required in order to oxidise starch.
Family Firsts
- First family member identified
- AA13 from A. oryzae [2].
- First demonstration of oxidative cleavage
- The N. crassa AA13 was shown to produce oxidised malto-oligosaccharides in the presence of oxygen and reducing agents [1].
- First 3-D structure
- AA13 from A. oryzae with Cu+ 4OPB [2]
References
- Vu VV, Beeson WT, Span EA, Farquhar ER, and Marletta MA. (2014). A family of starch-active polysaccharide monooxygenases. Proc Natl Acad Sci U S A. 2014;111(38):13822-7. DOI:10.1073/pnas.1408090111 |
- Lo Leggio L, Simmons TJ, Poulsen JC, Frandsen KE, Hemsworth GR, Stringer MA, von Freiesleben P, Tovborg M, Johansen KS, De Maria L, Harris PV, Soong CL, Dupree P, Tryfona T, Lenfant N, Henrissat B, Davies GJ, and Walton PH. (2015). Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat Commun. 2015;6:5961. DOI:10.1038/ncomms6961 |
-
Nekiunaite, L., Arntzen, M.Ø., Svensson, B., Vaaje-Kolstad, G., Abou Hachem, M. (2016) Lytic polysaccharide monooxygenases and other oxidative enzymes are abundantly secreted by Aspergillus nidulans grown on different starches. Biotechnology for Biofuels. 9, 187 [1]
- Vu VV and Marletta MA. (2016). Starch-degrading polysaccharide monooxygenases. Cell Mol Life Sci. 2016;73(14):2809-19. DOI:10.1007/s00018-016-2251-9 |
- Walton PH and Davies GJ. (2016). On the catalytic mechanisms of lytic polysaccharide monooxygenases. Curr Opin Chem Biol. 2016;31:195-207. DOI:10.1016/j.cbpa.2016.04.001 |
- Kim S, Ståhlberg J, Sandgren M, Paton RS, and Beckham GT. (2014). Quantum mechanical calculations suggest that lytic polysaccharide monooxygenases use a copper-oxyl, oxygen-rebound mechanism. Proc Natl Acad Sci U S A. 2014;111(1):149-54. DOI:10.1073/pnas.1316609111 |
- Bertini L, Breglia R, Lambrughi M, Fantucci P, De Gioia L, Borsari M, Sola M, Bortolotti CA, and Bruschi M. (2018). Catalytic Mechanism of Fungal Lytic Polysaccharide Monooxygenases Investigated by First-Principles Calculations. Inorg Chem. 2018;57(1):86-97. DOI:10.1021/acs.inorgchem.7b02005 |
- Nekiunaite L, Isaksen T, Vaaje-Kolstad G, and Abou Hachem M. (2016). Fungal lytic polysaccharide monooxygenases bind starch and β-cyclodextrin similarly to amylolytic hydrolases. FEBS Lett. 2016;590(16):2737-47. DOI:10.1002/1873-3468.12293 |
- Vaaje-Kolstad G, Forsberg Z, Loose JS, Bissaro B, and Eijsink VG. (2017). Structural diversity of lytic polysaccharide monooxygenases. Curr Opin Struct Biol. 2017;44:67-76. DOI:10.1016/j.sbi.2016.12.012 |
- Forsberg Z, Mackenzie AK, Sørlie M, Røhr ÅK, Helland R, Arvai AS, Vaaje-Kolstad G, and Eijsink VG. (2014). Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases. Proc Natl Acad Sci U S A. 2014;111(23):8446-51. DOI:10.1073/pnas.1402771111 |
- Borisova AS, Isaksen T, Dimarogona M, Kognole AA, Mathiesen G, Várnai A, Røhr ÅK, Payne CM, Sørlie M, Sandgren M, and Eijsink VG. (2015). Structural and Functional Characterization of a Lytic Polysaccharide Monooxygenase with Broad Substrate Specificity. J Biol Chem. 2015;290(38):22955-69. DOI:10.1074/jbc.M115.660183 |
- Span EA, Suess DLM, Deller MC, Britt RD, and Marletta MA. (2017). The Role of the Secondary Coordination Sphere in a Fungal Polysaccharide Monooxygenase. ACS Chem Biol. 2017;12(4):1095-1103. DOI:10.1021/acschembio.7b00016 |
- Forsberg Z, Bissaro B, Gullesen J, Dalhus B, Vaaje-Kolstad G, and Eijsink VGH. (2018). Structural determinants of bacterial lytic polysaccharide monooxygenase functionality. J Biol Chem. 2018;293(4):1397-1412. DOI:10.1074/jbc.M117.817130 |