CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolase Family 93"
Harry Brumer (talk | contribs) |
|||
| Line 62: | Line 62: | ||
</biblio> | </biblio> | ||
<!-- DO NOT REMOVE THIS CATEGORY TAG! (...but please delete the nowiki tags before saving.) --> | <!-- DO NOT REMOVE THIS CATEGORY TAG! (...but please delete the nowiki tags before saving.) --> | ||
| − | [[Category:Glycoside Hydrolase Families]] | + | [[Category:Glycoside Hydrolase Families|GH093]] |
Revision as of 11:31, 30 July 2009
| Glycoside Hydrolase Family GH93 | |
| Clan | GH-E |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| http://www.cazy.org/fam/GH93.html | |
Substrate specificities
The characterized member of family 93 are known to hydrolyse linear α-1,5-L-arabinan. [1], [2], EC:3.2.1-
Kinetics and Mechanism
GH93 enzymes are exoenzymes which only release arabinobiose from the non-reducing end. Net retention of the configuration of the anomeric carbon is proposed from the products of the transglycosylation activity of the protein Abnx from Penicillium chrysogenum. [3] It was recently supported in the crystal structure of the Arb93A enzyme from Fusarium graminearum in complex with arabinobiose, the degradation product of alpha-methyl-arabinotetraose. [2]
Catalytic Residues
From the crystal structure of Arb93A, Glu170 and Glu242 are proposed to act as nucleophile and acid/base respectively. Mutagenesis experiment support their role in catalysis and they are strictly conserved between the family members. [2]
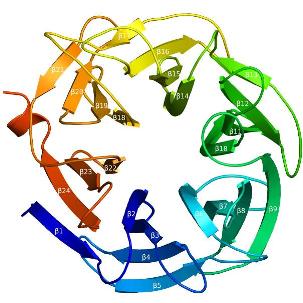
Three-dimensional structures
The recent crystal structure of Arb93A reveals a six-bladed beta-propeller fold characteristic of sialidases of clan GHE. [2], [4] The catalytic machinery is however very different from that of sialidases.
Family Firsts
First sterochemistry determination
This was determined with the Abxn enzyme using the H1-NMR technique to follow the products of its transglycosylation activity [3]
First catalytic nucleophile identification This was proposed based on the structure of Arb93A [2]
First general acid/base residue identification This was proposed based on the structure of Arb93A [2]
First 3-D structure Determined for Arb93A by Carapito and co-workers [2]
References
- Sakamoto T and Thibault JF. (2001). Exo-arabinanase of Penicillium chrysogenum able to release arabinobiose from alpha-1,5-L-arabinan. Appl Environ Microbiol. 2001;67(7):3319-21. DOI:10.1128/AEM.67.7.3319-3321.2001 |
- Carapito R, Imberty A, Jeltsch JM, Byrns SC, Tam PH, Lowary TL, Varrot A, and Phalip V. (2009). Molecular basis of arabinobio-hydrolase activity in phytopathogenic fungi: crystal structure and catalytic mechanism of Fusarium graminearum GH93 exo-alpha-L-arabinanase. J Biol Chem. 2009;284(18):12285-96. DOI:10.1074/jbc.M900439200 |
- Sakamoto T, Fujita T, and Kawasaki H. (2004). Transglycosylation catalyzed by a Penicillium chrysogenum exo-1,5-alpha-L-arabinanase. Biochim Biophys Acta. 2004;1674(1):85-90. DOI:10.1016/j.bbagen.2004.06.001 |
- Gaskell A, Crennell S, and Taylor G. (1995). The three domains of a bacterial sialidase: a beta-propeller, an immunoglobulin module and a galactose-binding jelly-roll. Structure. 1995;3(11):1197-205. DOI:10.1016/s0969-2126(01)00255-6 |