CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Transglycosylases"
| (27 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
== Overview == | == Overview == | ||
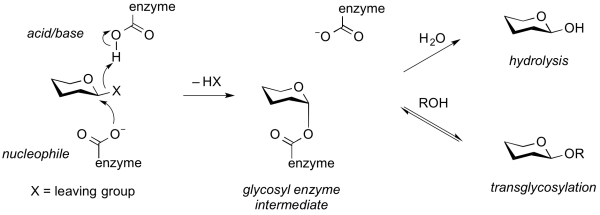
| − | '''Transglycosylases''' are a class of GH enzymes that can catalyze the transformation of one glycoside to another. That is, these enzymes catalyze the | + | '''Transglycosylases''' (also transglycosidases) are a class of GH enzymes that can catalyze the transformation of one glycoside to another. That is, these enzymes catalyze the intra- or intermolecular substitution of the anomeric position of a glycoside. Mechanistically, transglycosylases utilize the same mechanism as various [[retaining]] glycoside hydrolases <cite>Sinnott1990 Bissaro2015</cite>. Thus, reaction of the nucleophile of a retaining glycoside hydrolase with a substrate gives a glycosyl-enzyme [[intermediate]] that can be intercepted either by water to give the hydrolysis product, or by another acceptor (often another carbohydrate alcohol), to give a new glycoside or oligosaccharide <cite>Crout1998</cite>. Alternatively, transglycosylation can occur by [[neighboring group participation]], wherein a neighboring 2-acetamido group participates in the reaction to generate an [[oxazolinium ion]] intermediate, which again can react with another acceptor other than water. Some transglycosidases possess substantial [[glycoside hydrolase]] activity, and some glycoside hydrolases possess transglycosylase activity <cite>Vocadlo2000</cite>. Indeed, in many cases it is unclear what the major role of an enzyme that possesses both activities may be. Transglycosylases are [[sequence-based classification|classified]] as [[glycoside hydrolases]] into various GH families on the basis of sequence similarity. |
| − | [[Image:transglycosylation.png|600px| | + | [[Image:transglycosylation.png|thumb|center|600px|'''Figure. Generalized mechanism of a transglycosylase.''' Enzymatic cleavage of a substrate through a [[classical Koshland retaining mechanism]] results in formation of a glycosyl enzyme intermediate. This can partition to react with either water to cause hydrolysis ([[glycoside hydrolase]] activity) or to an alternative acceptor, often a sugar, to cause transglycosylation (transglycosylase activity).]] |
| − | |||
===Families=== | ===Families=== | ||
| + | GH families with notable transglycosylase activity include:<br> | ||
| + | *[[GH2]], for example LacZ β-galactosidase converts lactose to allolactose <cite>Juers2012</cite>.<br> | ||
| + | *[[GH13]], for example cyclodextran glucanotransferases that convert linear amylose to cyclodextrins <cite>Uitdehaag1999</cite>; glycogen debranching enzyme, which transfers three glucose residues from the four-residue glycogen branch to a nearby branch <cite>Braun1996</cite>; and trehalose synthase, which catalyzes the interconversion of maltose and trehalose <cite>Zhang2011</cite>.<br> | ||
| + | *[[GH16]], for example xyloglucan endotransglycosylases, which cuts and rejoins xyloglucan chains in the plant cell wall <cite>Eklof2010</cite>. | ||
| + | *[[GH31]], for example α-transglucosidases, which catalyze the transfer of individual glucosyl residues between α-(1→4)-glucans <cite>Larsbrink2012</cite>. | ||
| + | *[[GH70]], for example glucansucrases, which catalyse the synthesis of high molecular weight glucans, from sucrose <cite>Hijum2006</cite>. | ||
| + | *[[GH77]], for examples amylomaltase, which catalyzes the synthesis of maltodextrins from maltose <cite>Maarel2013</cite>. | ||
| + | *[[GH23]], [[GH102]], [[GH103]], and [[GH104]] lytic transglycosylases, which convert peptidoglycan to 1,6-anhydrosugars <cite>Schuerwater2008</cite>. | ||
| − | + | ===Inverting transglycosylases=== | |
| − | + | An anomer-inverting transglycosylase has been reported that is a member of Family [[GH186]], namely the cyclic glucohexadecaose-producing enzyme from ''Xanthomonas'' sp. <cite>#Motouchi2024</cite>. This enzyme converts linear β-1,2-glucan to cyclic-(β-1,2-Glc)<sub>15</sub>-1,6α. The reaction involves transglycosylation of a β-1,2-Glc bond to generate an α-1,6-Glc bond. Enzymes of this family are inverting glycosidases, and presumably, this product will be a substrate for the enzyme, possibly reversing the reaction or catalyzing hydrolysis, although this was not studied. | |
| − | |||
| − | |||
== References == | == References == | ||
<biblio> | <biblio> | ||
#Crout1998 pmid=9667913 | #Crout1998 pmid=9667913 | ||
| − | + | #Larsbrink2012 pmid=23132856 | |
| + | #Juers2012 pmid=23011886 | ||
| + | #Eklof2010 pmid=20421457 | ||
| + | #Braun1996 pmid=8611536 | ||
| + | #Uitdehaag1999 pmid=10331869 | ||
| + | #Hijum2006 pmid=16524921 | ||
| + | #Zhang2011 pmid=21840994 | ||
| + | #Schuerwater2008 pmid=17468031 | ||
| + | #Maarel2013 pmid=23465909 | ||
| + | #Vocadlo2000 Vocadlo, D. J. and Withers, S. G. (2008) Glycosidase-Catalysed Oligosaccharide Synthesis, Chapter 29 in ''Carbohydrates in Chemistry and Biology'', Ernst, B., Hart, G. W. and Sinaý, P., eds., Wiley-VCH Verlag GmbH, Weinheim, Germany. [http://dx.doi.org/10.1002/9783527618255.ch29 DOI:10.1002/9783527618255.ch29] | ||
| + | #Sinnott1990 Sinnott, M.L. (1990) Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 90, 1171-1202. [http://dx.doi.org/10.1021/cr00105a006 DOI: 10.1021/cr00105a006] | ||
| + | #Bissaro2015 pmid=25793417 | ||
| + | #Motouchi2024 pmid=38957137 | ||
| + | |||
</biblio> | </biblio> | ||
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Latest revision as of 16:58, 1 August 2024
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
Overview
Transglycosylases (also transglycosidases) are a class of GH enzymes that can catalyze the transformation of one glycoside to another. That is, these enzymes catalyze the intra- or intermolecular substitution of the anomeric position of a glycoside. Mechanistically, transglycosylases utilize the same mechanism as various retaining glycoside hydrolases [1, 2]. Thus, reaction of the nucleophile of a retaining glycoside hydrolase with a substrate gives a glycosyl-enzyme intermediate that can be intercepted either by water to give the hydrolysis product, or by another acceptor (often another carbohydrate alcohol), to give a new glycoside or oligosaccharide [3]. Alternatively, transglycosylation can occur by neighboring group participation, wherein a neighboring 2-acetamido group participates in the reaction to generate an oxazolinium ion intermediate, which again can react with another acceptor other than water. Some transglycosidases possess substantial glycoside hydrolase activity, and some glycoside hydrolases possess transglycosylase activity [4]. Indeed, in many cases it is unclear what the major role of an enzyme that possesses both activities may be. Transglycosylases are classified as glycoside hydrolases into various GH families on the basis of sequence similarity.
Families
GH families with notable transglycosylase activity include:
- GH2, for example LacZ β-galactosidase converts lactose to allolactose [5].
- GH13, for example cyclodextran glucanotransferases that convert linear amylose to cyclodextrins [6]; glycogen debranching enzyme, which transfers three glucose residues from the four-residue glycogen branch to a nearby branch [7]; and trehalose synthase, which catalyzes the interconversion of maltose and trehalose [8].
- GH16, for example xyloglucan endotransglycosylases, which cuts and rejoins xyloglucan chains in the plant cell wall [9].
- GH31, for example α-transglucosidases, which catalyze the transfer of individual glucosyl residues between α-(1→4)-glucans [10].
- GH70, for example glucansucrases, which catalyse the synthesis of high molecular weight glucans, from sucrose [11].
- GH77, for examples amylomaltase, which catalyzes the synthesis of maltodextrins from maltose [12].
- GH23, GH102, GH103, and GH104 lytic transglycosylases, which convert peptidoglycan to 1,6-anhydrosugars [13].
Inverting transglycosylases
An anomer-inverting transglycosylase has been reported that is a member of Family GH186, namely the cyclic glucohexadecaose-producing enzyme from Xanthomonas sp. [14]. This enzyme converts linear β-1,2-glucan to cyclic-(β-1,2-Glc)15-1,6α. The reaction involves transglycosylation of a β-1,2-Glc bond to generate an α-1,6-Glc bond. Enzymes of this family are inverting glycosidases, and presumably, this product will be a substrate for the enzyme, possibly reversing the reaction or catalyzing hydrolysis, although this was not studied.
References
Error fetching PMID 23132856:
Error fetching PMID 23011886:
Error fetching PMID 20421457:
Error fetching PMID 8611536:
Error fetching PMID 10331869:
Error fetching PMID 16524921:
Error fetching PMID 21840994:
Error fetching PMID 17468031:
Error fetching PMID 23465909:
Error fetching PMID 25793417:
Error fetching PMID 38957137:
-
Sinnott, M.L. (1990) Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 90, 1171-1202. DOI: 10.1021/cr00105a006
- Error fetching PMID 25793417:
- Error fetching PMID 9667913:
-
Vocadlo, D. J. and Withers, S. G. (2008) Glycosidase-Catalysed Oligosaccharide Synthesis, Chapter 29 in Carbohydrates in Chemistry and Biology, Ernst, B., Hart, G. W. and Sinaý, P., eds., Wiley-VCH Verlag GmbH, Weinheim, Germany. DOI:10.1002/9783527618255.ch29
- Error fetching PMID 23011886:
- Error fetching PMID 10331869:
- Error fetching PMID 8611536:
- Error fetching PMID 21840994:
- Error fetching PMID 20421457:
- Error fetching PMID 23132856:
- Error fetching PMID 16524921:
- Error fetching PMID 23465909:
- Error fetching PMID 17468031:
- Error fetching PMID 38957137: