CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Carbohydrate Binding Module Family 105"
| Line 18: | Line 18: | ||
== Ligand specificities == | == Ligand specificities == | ||
| − | The first member of family CBM105 (SoCBM) was identified in the [[PL29]] multidomain chondroitinase ChABC29So from ''Segatella oris'' <cite>Liu2024</cite>. SoCBM bound specifically to chondroitin sulfates (CSs) including CS-A and CS-C, while incapable of binding to other | + | The first member of family CBM105 (SoCBM) was identified in the [[PL29]] multidomain chondroitinase ChABC29So from ''Segatella oris'' <cite>Liu2024</cite>. SoCBM bound specifically to chondroitin sulfates (CSs) including CS-A and CS-C, while it was incapable of binding to other glycosaminoglycans or polyuronic acid substrates. |
== Structural Features == | == Structural Features == | ||
Revision as of 07:31, 6 November 2024
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| https://www.cazy.org/CBM105.html |
Ligand specificities
The first member of family CBM105 (SoCBM) was identified in the PL29 multidomain chondroitinase ChABC29So from Segatella oris [1]. SoCBM bound specifically to chondroitin sulfates (CSs) including CS-A and CS-C, while it was incapable of binding to other glycosaminoglycans or polyuronic acid substrates.
Structural Features
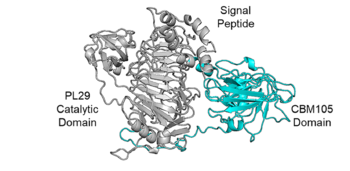
An AlphaFold2 model predicts that SoCBM has a β-sandwich fold (Fig.1).
Functionalities
SoCBM is the C-terminus domain of a PL29 enzyme ChABC29So that displays chondroitin sulfate ABC activity, consistent with the SoCBM specificity. Biochemical characterization of ChABC29So and the CBM-truncated enzyme revealed that the SoCBM enhances the catalytic activity, thermostability, and disaccharide proportion in the final enzymatic products of ChABC29So.
Family Firsts
- First Identified
- The chondroitin sulfate binding SoCBM from the S. oris PL29 chondroitinase was the first member of the family to be identified and characterized[1].
- First Structural Characterization
- No experimentally determined three-dimensional structure has been solved in this CBM family.