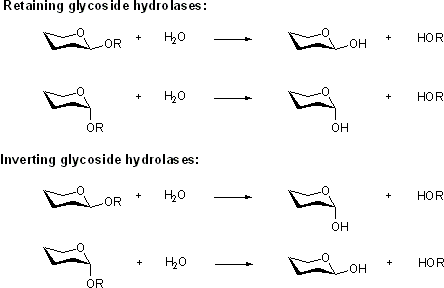CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside hydrolases"
Harry Brumer (talk | contribs) m |
|||
| Line 1: | Line 1: | ||
| − | + | * Authors: ^^^Steve Withers^^^, ^^^Spencer Williams^^^ | |
| − | + | * Responsible Curator: ^^^Spencer Williams^^^ | |
| − | * Authors: | ||
| − | * Responsible Curator: | ||
---- | ---- | ||
== Overview == | == Overview == | ||
Revision as of 04:08, 29 September 2009
- Authors: ^^^Steve Withers^^^, ^^^Spencer Williams^^^
- Responsible Curator: ^^^Spencer Williams^^^
Overview
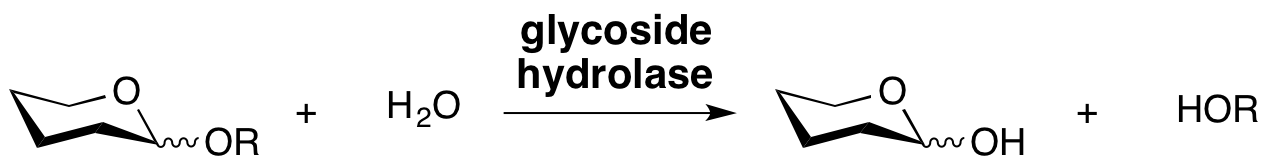
Glycoside hydrolases are enzymes that catalyze the hydrolysis of the glycosidic linkage of glycosides, leading to the formation of a sugar hemiacetal or hemiketal and the corresponding free aglycon. Glycoside hydrolases are also referred to as glycosidases, and sometimes also as glycosyl hydrolases. Glycoside hydrolases can catalyze the hydrolysis of O-, N- and S-linked glycosides.
Classification
Glycoside hydrolases can be classified in many different ways. The following paragraphs list several different ways, the utility of which depends on the context in which the classification is made and used.
Endo/exo
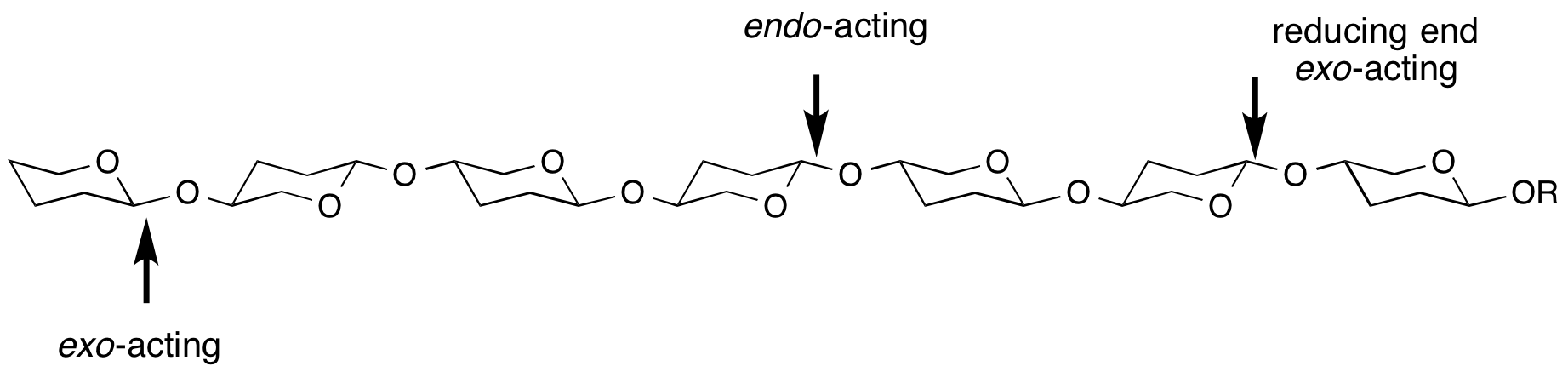
exo- and endo- refers to the ability of a glycoside hydrolase to cleave a substrate at the end (most frequently, but not always the non-reducing end) or within the middle of a chain. For example, most cellulases areendo-acting, whereas LacZ β-galactosidase from E. coli is exo-acting.
Enzyme Commission (EC) number
EC numbers are codes representing the Enzyme Commission number. This is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze. Every EC number is associated with a recommended name for the respective enzyme. EC numbers do not specify enzymes, but enzyme-catalyzed reactions. If different enzymes (for instance from different organisms) catalyze the same reaction, then they receive the same EC number. A necessary consequence of the EC classification scheme is that codes can be applied only to enzymes for which a function has been biochemically identified. Additionally, certain enzymes can catalyze reactions that fall in more than one class. These enzymes must bear more than one EC number.
Mechanistic classification
Two reaction mechanisms are most commonly found for the retaining and inverting enzymes, as first outlined by Koshland and as described below.[1] However several interesting variations on these mechanisms have been found, and one fundamentally different mechanism, catalyzed by an NADH cofactor, has been discovered in recent years, as discussed below.
Sequence classification
Sequence classification methods require knowledge of at least part of the amino acid sequence for an enzyme. Algorithmic methods are then used to compare sequences, and in the case of the glycoside hydrolases, this has allowed their classification into more than 100 families. Each family (GH family) contains proteins that are related by sequence, and by corollary, fold. An obvious shortcoming of sequence-based classifications is that they can only be applied to enzymes for which sequence information is available. On the other hand sequence-based classification schemes allow classification of proteins for which no biochemical evidence has been obtained such as the thousands of uncharacterized glycosidase-related sequences that orginate from genome sequencing efforts worldwide. This allows a number of useful predictions to be made since it has long been noted that the catalytic machinery and molecular mechanism is conserved for the vast majority of the glycosidase families [1] as well as the geometry around the glycosidic bond (irrespective of naming conventions) [2]. As such sequence based classification methods are rather different (and in many ways complimentary) to the EC method discussed above.
Mechanism
Two reaction mechanisms are most commonly found for the retaining and inverting enzymes, as first outlined by Koshland and as described below [3]. However several interesting variations on these mechanisms have been found, and one fundamentally different mechanism, catalyzed by an NADH cofactor, has been discovered in recent years.
Inverting glycoside hydrolases
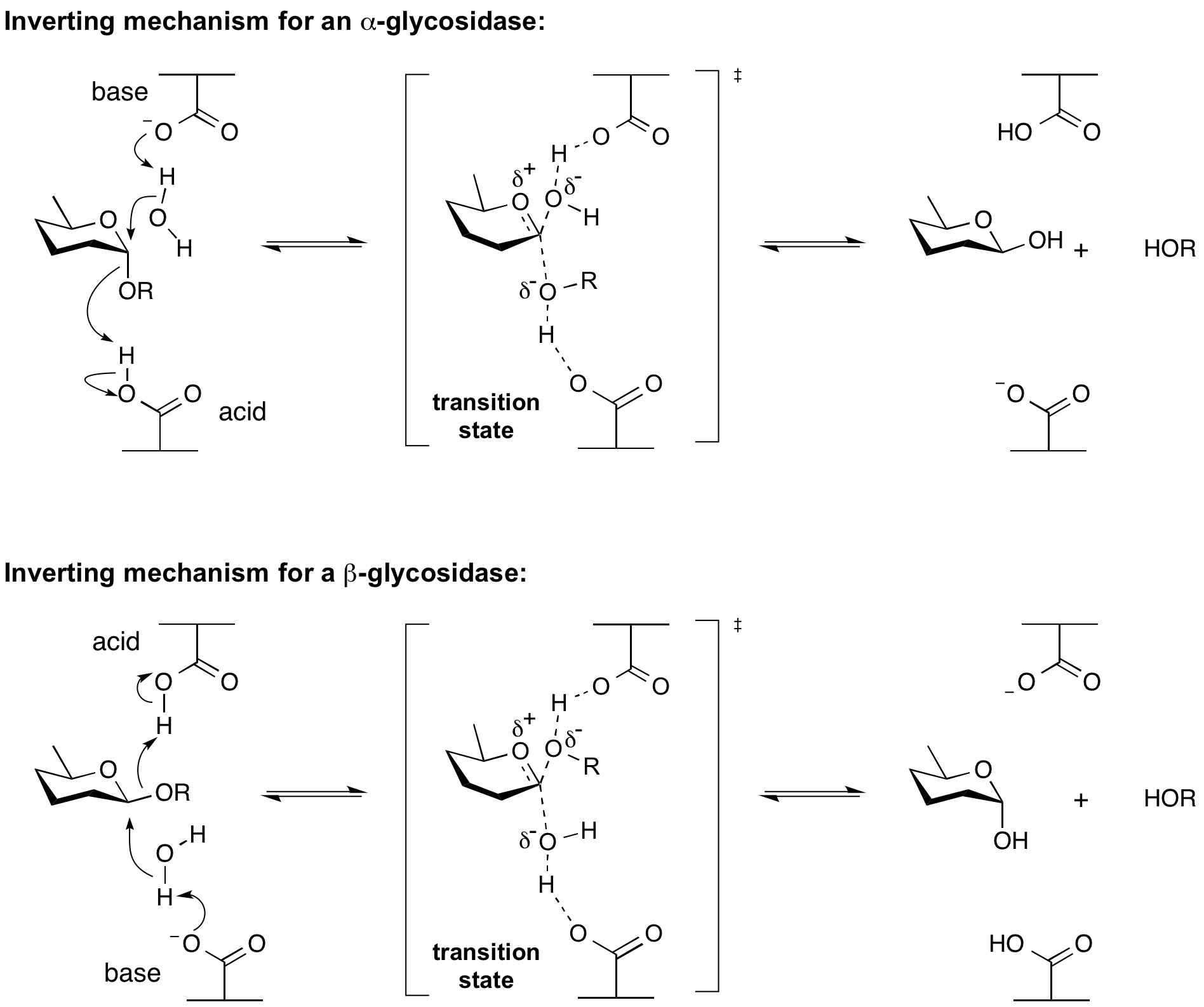
Hydrolysis of a glycoside with net inversion of anomeric configuration is generally achieved via a one step, single-displacement mechanism involving oxocarbenium ion-like transition states, as shown below. The reaction occurs with acid/base assistance from two amino acid side chains, normally glutamic or aspartic acids, that are typically located 6-11 A apart[4].
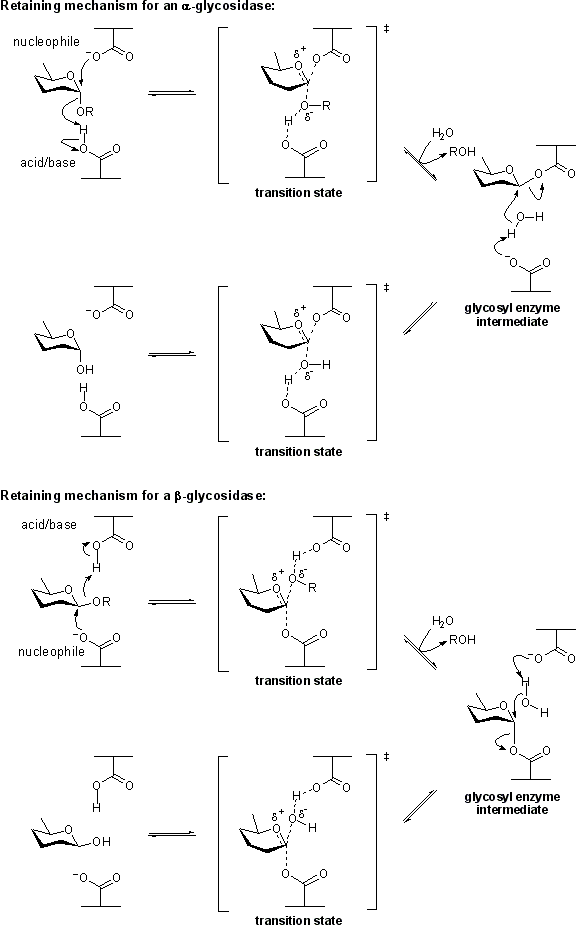
Retaining glycoside hydrolases
Classical Koshland retaining mechanism
Hydrolysis with net retention of configuration is most commonly achieved via a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate, as is shown in the figure below. Each step passes through anoxocarbenium ion-like transition state. Reaction occurs with acid/base and nucleophilic assistance provided by two amino acid side chains, typically glutamate or aspartate, located 5.5 A apart. In the first step (often called the glycosylation step), one residue plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a base catalyst deprotonating the water molecule as it attacks. The pKa value of the acid/base group cycles between high and low values during catalysis to optimize it for its role at each step of catalysis [5]. In the case of sialidases, the catalytic nucleophile is a tyrosine residue (see below). This mechanism was originally proposed by Dan Koshland, although at the time the identities of the residues was unclear [3].
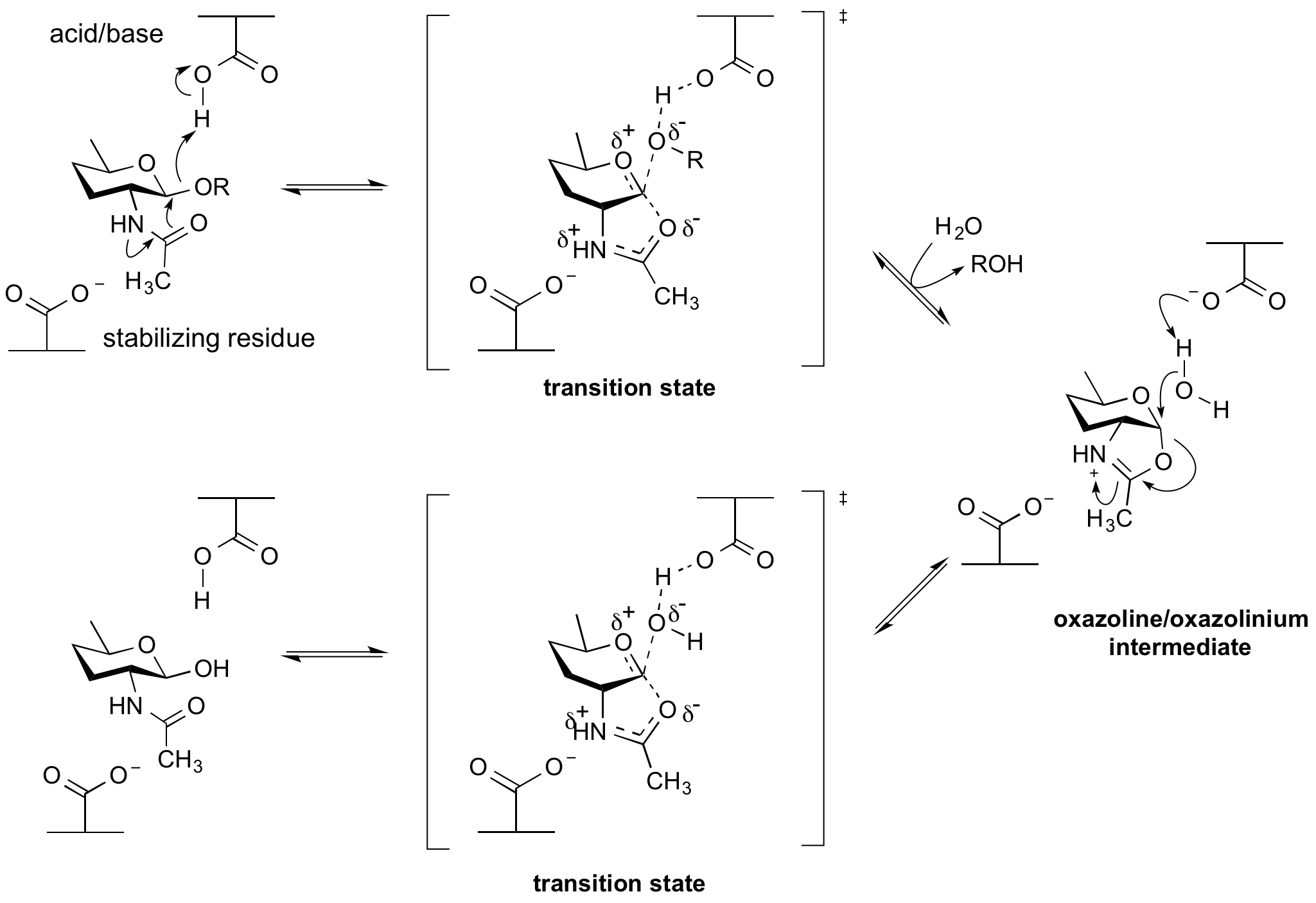
Neighboring group participation
Enzymes of glycoside hydrolase families 18, 20,25, 56, 84, and85 hydrolyse substrates containing an N-acetyl (acetamido) or N-glycolyl group at the 2-position. These enzymes have no catalytic nucleophile: rather they utilize a mechanism in which the 2-acetamido group acts as an intramolecular nucleophile. Neighboring group participation by the 2-acetamido group leads to formation of an oxazoline (or more strictly an oxazolinium ion) intermediate. This mechanism was deduced from X-ray structures of complexes of chitinases with natural inhibitors [6], from the potent inhibition afforded by a stable thiazoline analogue of the oxazoline [7][8], and from detailed mechanistic analyses using substrates of modified reactivity [9]. Typically, a stabilizing residue (a carboxylate) stabilizes the charge development in the transition state. Not all enzymes that cleave substrates possessing a 2-acetamido group utilize a neighboring groups participation mechanism; enzyme of glycoside hydrolase families 3 and 22 utilize a classical retaining mechanism with an enzymic nucleophile. Other hexosaminidases such as those of Glycoside Hydrolase Family 19 utilize an inverting mechanism.
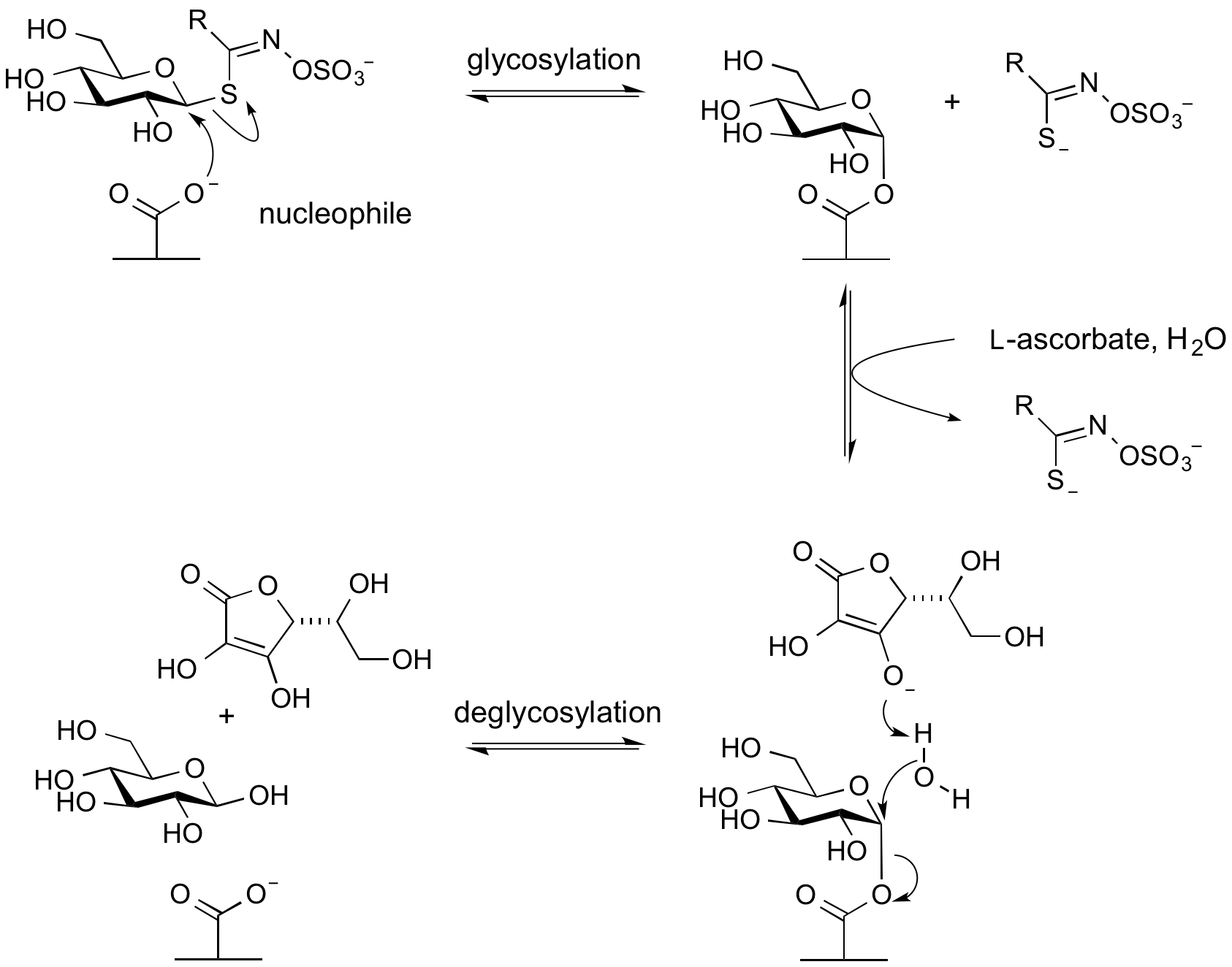
Exogenous bases: Myrosinases
Glycoside hydrolases termed myrosinases catalyze the hydrolysis of anionic thioglycosides (glucosinolates) found in plants. They are found in Glycoside Hydrolase Family 1. In these enzymes the usual acid/base glutamic acid found in other members of this family is replaced by a glutamine. This likely reduces charge repulsion between the anionic aglycon sulfate. The unusual aglycon is a sufficiently good leaving group to be able to cleave in the first glycosylation step to form the glycosyl enzyme intermediate without the requirement of an acid catalyst. However, since a base catalyst is required for the second step (hydrolysis or deglycosylation) these enzymes require an alternative basic group. This is provided by the co-enzyme L-ascorbate [10].
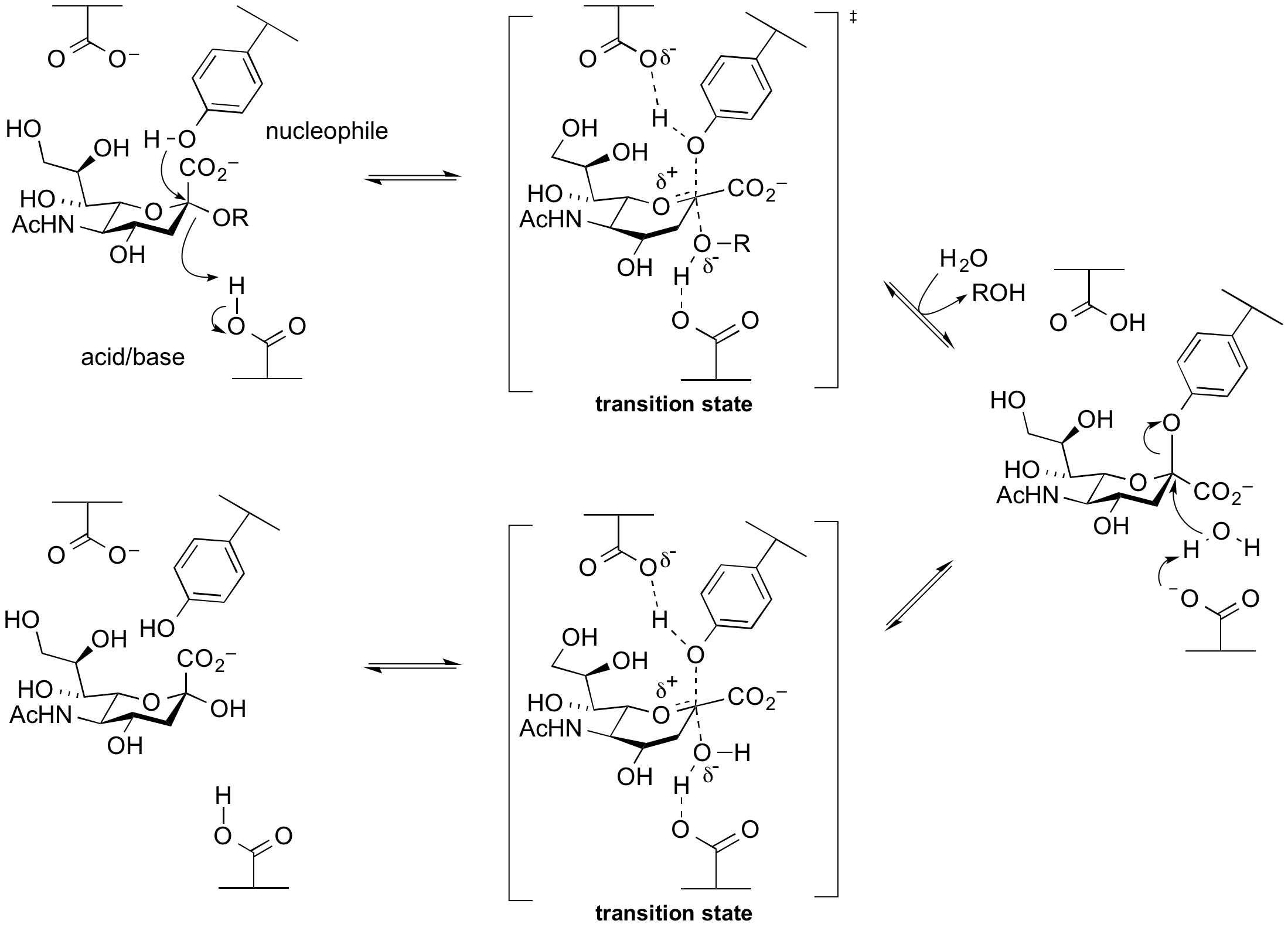
Alternative nucleophiles
Enzymes termed sialidases (also neuraminidases) hydrolyze glycosides of sialic acids. Closely related enzymes termed trans-sialidases catalyze the transglycosylation of sialisides. The sialidases and trans-sialidases of glycoside hydrolase families 33 and 34 utilize a tyrosine as a catalytic nucleophile, which is activated by an adjacent base residue. A rationale for this unusual difference is that the use of a negatively charged carboxylate as a nucelophile will be disfavoured as the anomeric centre is itself negatively charged, and thus charge repulsion interferes. A tyrosine residue is a neutral nucleophile, but requires a general base to enhance its nucleophilicity. This mechanism was implied from X-ray structures, and was supported by experiments involving trapping of the intermediate with fluorosugars followed by peptide mapping and then crystallography[11][12], as well as via mechanistic studies on mutants [13].
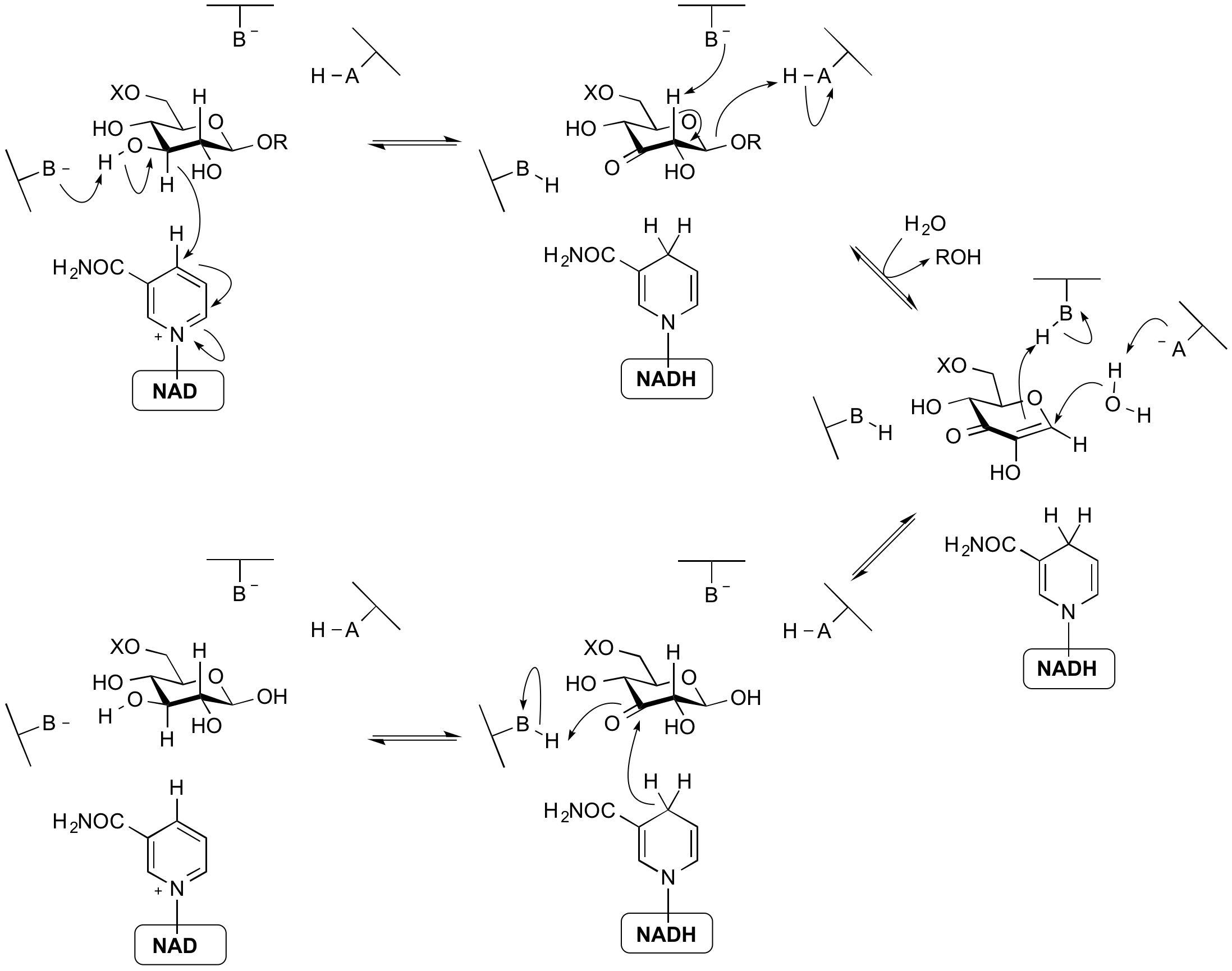
NAD-dependent hydrolysis
The glycoside hydrolases of family 4 and 109 use a mechanism that requires an NAD cofactor, which remains tightly bound throughout catalysis. The mechanism proceeds via anionic transition states with elimination and redox steps rather than the classical mechanisms proceeding throughoxocarbenium ion-like transition states. As shown below for a 6-phospho-beta-glucosidase, the mechanism involves an initial oxidation of the 3-hydroxyl of the substrate by the enzyme-bound NAD cofactor. This increases the acidity of the C2 proton such that an E1cb elimination can occur with assistance from an enzymatic base. The alpha-beta unsaturated intermediate formed then undergoes addition of water at the anomeric centre and finally the ketone at C3 is reduced to generate the free sugar product. Thus, even though glycosidic bond cleavage occurred via an elimination mechanism, the overall reaction is hydrolysis. This mechanism was elucidated through a combination of stereochemical studies by NMR, kinetic isotope effects, linear free energy relationships, X-ray crystallography and UV/Vis spectrophotometry [14, 15].
References
- Gebler J, Gilkes NR, Claeyssens M, Wilson DB, Béguin P, Wakarchuk WW, Kilburn DG, Miller RC Jr, Warren RA, and Withers SG. (1992). Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases. J Biol Chem. 1992;267(18):12559-61. | Google Books | Open Library
- Henrissat B, Callebaut I, Fabrega S, Lehn P, Mornon JP, and Davies G. (1995). Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc Natl Acad Sci U S A. 1995;92(15):7090-4. DOI:10.1073/pnas.92.15.7090 |
-
Koshland, D. (1953) Biol. Rev. 28, 416.
- McCarter JD and Withers SG. (1994). Mechanisms of enzymatic glycoside hydrolysis. Curr Opin Struct Biol. 1994;4(6):885-92. DOI:10.1016/0959-440x(94)90271-2 |
- McIntosh LP, Hand G, Johnson PE, Joshi MD, Körner M, Plesniak LA, Ziser L, Wakarchuk WW, and Withers SG. (1996). The pKa of the general acid/base carboxyl group of a glycosidase cycles during catalysis: a 13C-NMR study of bacillus circulans xylanase. Biochemistry. 1996;35(31):9958-66. DOI:10.1021/bi9613234 |
- Terwisscha van Scheltinga AC, Armand S, Kalk KH, Isogai A, Henrissat B, and Dijkstra BW. (1995). Stereochemistry of chitin hydrolysis by a plant chitinase/lysozyme and X-ray structure of a complex with allosamidin: evidence for substrate assisted catalysis. Biochemistry. 1995;34(48):15619-23. DOI:10.1021/bi00048a003 |
-
Knapp, S., Vocadlo, D., Gao, Z. N., Kirk, B., Lou, J. P., and Withers, S. G. (1996) Journal of the American Chemical Society 118, 6804-6805.
- Mark BL, Vocadlo DJ, Knapp S, Triggs-Raine BL, Withers SG, and James MN. (2001). Crystallographic evidence for substrate-assisted catalysis in a bacterial beta-hexosaminidase. J Biol Chem. 2001;276(13):10330-7. DOI:10.1074/jbc.M011067200 |
- Vocadlo DJ and Withers SG. (2005). Detailed comparative analysis of the catalytic mechanisms of beta-N-acetylglucosaminidases from families 3 and 20 of glycoside hydrolases. Biochemistry. 2005;44(38):12809-18. DOI:10.1021/bi051121k |
- Burmeister WP, Cottaz S, Rollin P, Vasella A, and Henrissat B. (2000). High resolution X-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J Biol Chem. 2000;275(50):39385-93. DOI:10.1074/jbc.M006796200 |
- Amaya MF, Watts AG, Damager I, Wehenkel A, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, and Alzari PM. (2004). Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure. 2004;12(5):775-84. DOI:10.1016/j.str.2004.02.036 |
- Watts AG, Damager I, Amaya ML, Buschiazzo A, Alzari P, Frasch AC, and Withers SG. (2003). Trypanosoma cruzi trans-sialidase operates through a covalent sialyl-enzyme intermediate: tyrosine is the catalytic nucleophile. J Am Chem Soc. 2003;125(25):7532-3. DOI:10.1021/ja0344967 |
- Watson JN, Dookhun V, Borgford TJ, and Bennet AJ. (2003). Mutagenesis of the conserved active-site tyrosine changes a retaining sialidase into an inverting sialidase. Biochemistry. 2003;42(43):12682-90. DOI:10.1021/bi035396g |
- Yip VL, Varrot A, Davies GJ, Rajan SS, Yang X, Thompson J, Anderson WF, and Withers SG. (2004). An unusual mechanism of glycoside hydrolysis involving redox and elimination steps by a family 4 beta-glycosidase from Thermotoga maritima. J Am Chem Soc. 2004;126(27):8354-5. DOI:10.1021/ja047632w |
- Rajan SS, Yang X, Collart F, Yip VL, Withers SG, Varrot A, Thompson J, Davies GJ, and Anderson WF. (2004). Novel catalytic mechanism of glycoside hydrolysis based on the structure of an NAD+/Mn2+ -dependent phospho-alpha-glucosidase from Bacillus subtilis. Structure. 2004;12(9):1619-29. DOI:10.1016/j.str.2004.06.020 |