CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Transition state"
Harry Brumer (talk | contribs) |
Harry Brumer (talk | contribs) m |
||
| Line 1: | Line 1: | ||
| + | {{CuratorApproved}} | ||
* Author: [[User:Withers|Stephen Withers]] | * Author: [[User:Withers|Stephen Withers]] | ||
* Responsible Curator: [[User:SpencerWilliams|Spencer Williams]] | * Responsible Curator: [[User:SpencerWilliams|Spencer Williams]] | ||
Revision as of 06:18, 17 January 2010
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Stephen Withers
- Responsible Curator: Spencer Williams
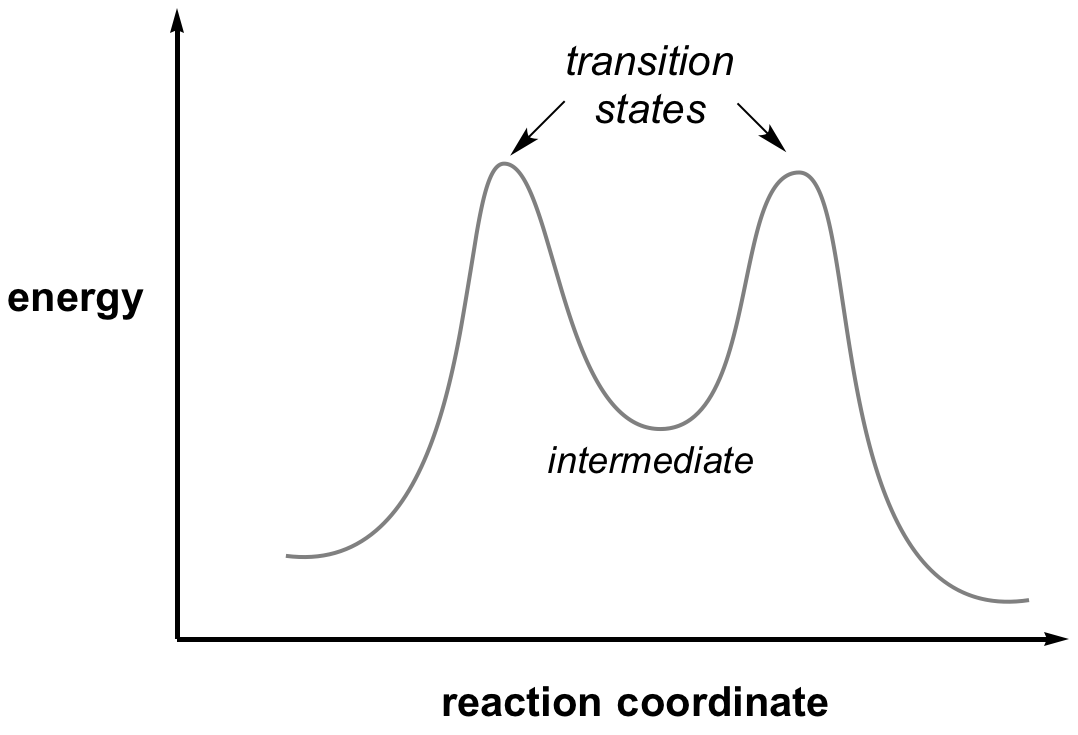
The transition state of any reaction is the highest point in the reaction coordinate, this is a balance point between starting material and product. By definition it does not have a lifetime, and so it cannot be isolated and studied directly. However, transition state structures can be determined by the measurement of kinetic isotope effects.