CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolase Family 117"
| Line 42: | Line 42: | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | At the moment two members of GH117 family have been crystallized. Both are enzymes from marine bacteria, one from ''Saccharophagus degradans'' <cite>Lee2009</cite> and one from ''Zobelia galactanivorans'' <cite>Rebuffet2011</cite>. A crystal structure has only been reported for the α-1,3-L-(3,6-anhydro)-galactosidase (AhgA,Zg4663) from ''Z. galactanivorans'' <cite>Rebuffet2011</cite>. | + | At the moment two members of GH117 family have been crystallized. Both are enzymes from marine bacteria, one from ''Saccharophagus degradans'' <cite>Lee2009</cite> and one from ''Zobelia galactanivorans'' <cite>Rebuffet2011</cite>. A crystal structure has only been reported for the α-1,3-L-(3,6-anhydro)-galactosidase (AhgA, Zg4663) from ''Z. galactanivorans'' (PDB: [http://www.pdb.org/pdb/explore/explore.do?structureId=3p2n 3P2N]) <cite>Rebuffet2011</cite>. |
| − | Zg4663 adopting the five-bladed β-propeller fold and form dimer via domain-swapping of the N-terminal HTH (Helix-Turn-Helix) domain (Figure )<cite>Rebuffet2011</cite>. | + | Zg4663 adopting the five-bladed β-propeller fold and form dimer via domain-swapping of the N-terminal HTH (Helix-Turn-Helix) domain (Figure 2) <cite>Rebuffet2011</cite>. Interestingly, previous sequences reported from Vibrio sp. JT0107 and Bacillus sp. MK03 contain the conserved domain-swapping signature SxAxxR in the HTH domain. Consistently, these proteins were reported to form multimers (a dimer and an octamer respectively), based on calibrated gel filtration estimations <cite>Sugano1994 Suzuki2002 </cite>. In contrast, RB13146 (Clade B) misses the domain-swapping signature, and the crucial residues are missing. This protein from R. baltica is thus likely a monomer and may represent an ‘ancestral’ form of the GH117 family which would be limited to the β-propeller, catalytic domain <cite>Rebuffet2011</cite>. |
| + | |||
| Line 60: | Line 61: | ||
#Rebuffet2011 pmid=21332624 | #Rebuffet2011 pmid=21332624 | ||
#Lee2009 pmid=20054134 | #Lee2009 pmid=20054134 | ||
| + | |||
#He1999 pmid=9312086 | #He1999 pmid=9312086 | ||
#StickWilliams isbn=978-0-240-52118-3 | #StickWilliams isbn=978-0-240-52118-3 | ||
Revision as of 04:08, 5 May 2011
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Etienne Rebuffet^^^
- Responsible Curator: ^^^Mirjam Czjzek^^^
| Glycoside Hydrolase Family GH117 | |
| Clan | None |
| Mechanism | Not known |
| Active site residues | Not known |
| CAZy DB link | |
| https://www.cazy.org/GH117.html | |
Substrate specificities
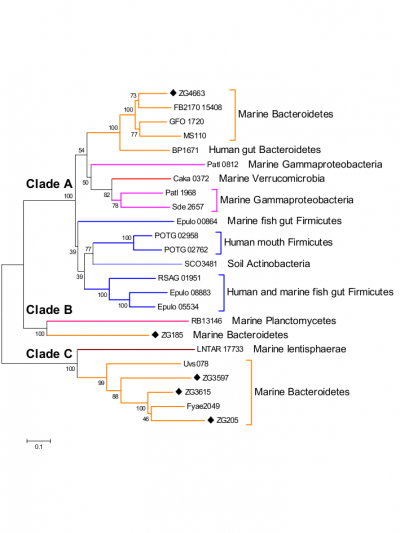
The only activity so far identified in this recently discovered family of glycoside hydrolases is that α-1,3-L-(3,6-anhydro)-galactosidase [1, 2, 3]. Nevertheless phylogenetic analysis (figure 1) of this family and activity test on Zg3597 (Clade C) show that the family GH117 is polyspecific [1].
Kinetics and Mechanism
Mechanism of glycoside hydrolase family 117 is still unknown. But structural analyse revealed the presence of a zinc ion close to the active site which could be involved in the reaction mechanism [1].
Catalytic Residues
From structural analysis and sequences alignment the catalytic residues have been predicted to be two out of the three acidic residues Asp-97, Asp-252 and Glu-310 (Zg4663 numbering) [1].
Three-dimensional structures
At the moment two members of GH117 family have been crystallized. Both are enzymes from marine bacteria, one from Saccharophagus degradans [4] and one from Zobelia galactanivorans [1]. A crystal structure has only been reported for the α-1,3-L-(3,6-anhydro)-galactosidase (AhgA, Zg4663) from Z. galactanivorans (PDB: 3P2N) [1]. Zg4663 adopting the five-bladed β-propeller fold and form dimer via domain-swapping of the N-terminal HTH (Helix-Turn-Helix) domain (Figure 2) [1]. Interestingly, previous sequences reported from Vibrio sp. JT0107 and Bacillus sp. MK03 contain the conserved domain-swapping signature SxAxxR in the HTH domain. Consistently, these proteins were reported to form multimers (a dimer and an octamer respectively), based on calibrated gel filtration estimations [2, 3]. In contrast, RB13146 (Clade B) misses the domain-swapping signature, and the crucial residues are missing. This protein from R. baltica is thus likely a monomer and may represent an ‘ancestral’ form of the GH117 family which would be limited to the β-propeller, catalytic domain [1].
Family Firsts
- First stereochemistry determination
- Cite some reference here, with a short (1-2 sentence) explanation [5].
- First catalytic nucleophile identification
- Cite some reference here, with a short (1-2 sentence) explanation [6].
- First general acid/base residue identification
- Cite some reference here, with a short (1-2 sentence) explanation [7].
- First 3-D structure
- Zg4663, α-1,3-L-(3,6-anhydro)-galactosidase (AhgA), PDB: 3P2N [1].
References
- Rebuffet E, Groisillier A, Thompson A, Jeudy A, Barbeyron T, Czjzek M, and Michel G. (2011). Discovery and structural characterization of a novel glycosidase family of marine origin. Environ Microbiol. 2011;13(5):1253-70. DOI:10.1111/j.1462-2920.2011.02426.x |
- Sugano Y, Kodama H, Terada I, Yamazaki Y, and Noma M. (1994). Purification and characterization of a novel enzyme, alpha-neoagarooligosaccharide hydrolase (alpha-NAOS hydrolase), from a marine bacterium, Vibrio sp. strain JT0107. J Bacteriol. 1994;176(22):6812-8. DOI:10.1128/jb.176.22.6812-6818.1994 |
- Suzuki H, Sawai Y, Suzuki T, and Kawai K. (2002). Purification and characterization of an extracellular alpha-neoagarooligosaccharide hydrolase from Bacillus sp. MK03. J Biosci Bioeng. 2002;93(5):456-63. DOI:10.1016/s1389-1723(02)80092-5 |
- Lee S, Lee JY, Ha SC, Jung J, Shin DH, Kim KH, and Choi IG. (2009). Crystallization and preliminary X-ray analysis of neoagarobiose hydrolase from Saccharophagus degradans 2-40. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65(Pt 12):1299-301. DOI:10.1107/S174430910904603X |
-
Sinnott, M.L. (1990) Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 90, 1171-1202. DOI: 10.1021/cr00105a006
- He S and Withers SG. (1997). Assignment of sweet almond beta-glucosidase as a family 1 glycosidase and identification of its active site nucleophile. J Biol Chem. 1997;272(40):24864-7. DOI:10.1074/jbc.272.40.24864 |
This is an example of how to make references to a journal article [5]. (See the References section below). Multiple references can go in the same place like this [5, 7]. You can even cite books using just the ISBN [8]. References that are not in PubMed can be typed in by hand [6].