CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Alpha-glucan lyases"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
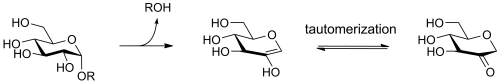
The alpha glucan lyases of [[Glycoside Hydrolase Family 31]] degrade starch via an elimination mechanism, rather than via hydrolysis, forming an unsaturated (enol) product that quickly tautomerises to its keto form, 1,5-anhydro fructose. | The alpha glucan lyases of [[Glycoside Hydrolase Family 31]] degrade starch via an elimination mechanism, rather than via hydrolysis, forming an unsaturated (enol) product that quickly tautomerises to its keto form, 1,5-anhydro fructose. | ||
| − | [[Image:A-glucan_lyase.png | + | [[Image:A-glucan_lyase.png|center|500px]] |
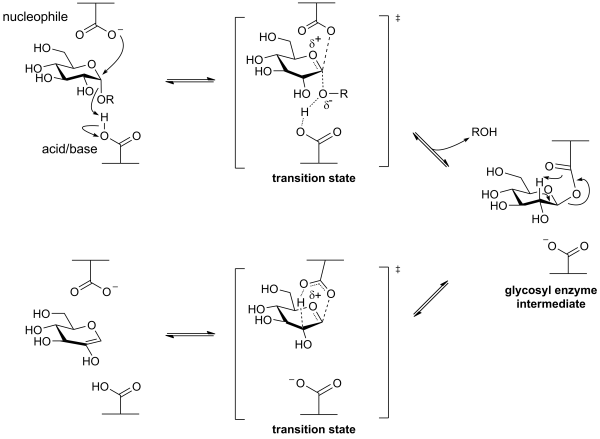
| − | The first half of their mechanism resembles that of [[Glycoside Hydrolase Family 31]] retaining alpha-glycosidases, and involves the formation of a covalent glycosyl-enzyme [[intermediate]]. Instead of hydrolyzing, this intermediate then decomposes through a syn-elimination mechanism, again via [[oxocarbenium ion]]-like [[transition state]]s, as shown below. This mechanism was demonstrated through a combination of studies involving [[intermediate]] trapping, kinetic isotope effects and linear free energy relationships <cite> | + | The first half of their mechanism resembles that of [[Glycoside Hydrolase Family 31]] retaining alpha-glycosidases, and involves the formation of a covalent glycosyl-enzyme [[intermediate]]. Instead of hydrolyzing, this intermediate then decomposes through a syn-elimination mechanism, again via [[oxocarbenium ion]]-like [[transition state]]s, as shown below. This mechanism was demonstrated through a combination of studies involving [[intermediate]] trapping, kinetic isotope effects and linear free energy relationships <cite>Lee2003 Yip2004</cite>. |
| − | [[Image:a-glucan lyase mechanism.png | + | [[Image:a-glucan lyase mechanism.png|center|600px]] |
== References == | == References == | ||
<biblio> | <biblio> | ||
| − | # | + | #Lee2003 pmid=14596624 |
| − | # | + | #Yip2004 pmid=15237973 |
</biblio> | </biblio> | ||
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Latest revision as of 03:44, 30 April 2013
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Stephen Withers
- Responsible Curator: Spencer Williams
The alpha glucan lyases of Glycoside Hydrolase Family 31 degrade starch via an elimination mechanism, rather than via hydrolysis, forming an unsaturated (enol) product that quickly tautomerises to its keto form, 1,5-anhydro fructose.
The first half of their mechanism resembles that of Glycoside Hydrolase Family 31 retaining alpha-glycosidases, and involves the formation of a covalent glycosyl-enzyme intermediate. Instead of hydrolyzing, this intermediate then decomposes through a syn-elimination mechanism, again via oxocarbenium ion-like transition states, as shown below. This mechanism was demonstrated through a combination of studies involving intermediate trapping, kinetic isotope effects and linear free energy relationships [1, 2].
References
- Lee SS, Yu S, and Withers SG. (2003). Detailed dissection of a new mechanism for glycoside cleavage: alpha-1,4-glucan lyase. Biochemistry. 2003;42(44):13081-90. DOI:10.1021/bi035189g |
- Yip VL, Varrot A, Davies GJ, Rajan SS, Yang X, Thompson J, Anderson WF, and Withers SG. (2004). An unusual mechanism of glycoside hydrolysis involving redox and elimination steps by a family 4 beta-glycosidase from Thermotoga maritima. J Am Chem Soc. 2004;126(27):8354-5. DOI:10.1021/ja047632w |