CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Absolute configuration (D/L nomenclature)"
(New page: * Author: Stephen Withers * Responsible Curator: Spencer Williams ---- The absolute configuration of all monosaccharides is denoted by ...) |
Harry Brumer (talk | contribs) m (updated citation keys to AuthorYear format) |
||
| (26 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{CuratorApproved}} | |
| − | |||
* [[Author]]: [[User:Withers|Stephen Withers]] | * [[Author]]: [[User:Withers|Stephen Withers]] | ||
* [[Responsible Curator]]: [[User:SpencerWilliams|Spencer Williams]] | * [[Responsible Curator]]: [[User:SpencerWilliams|Spencer Williams]] | ||
---- | ---- | ||
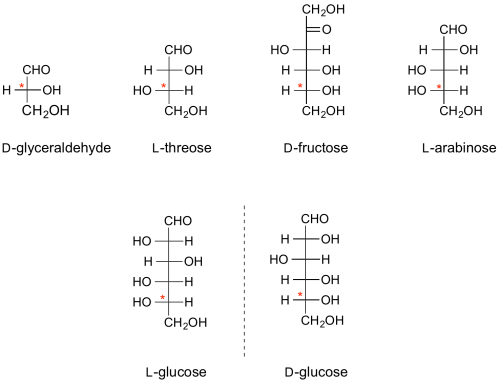
| − | The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation). If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals. | + | |
| + | The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation) <cite>StickWilliams2009</cite>. If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals. | ||
The configurations of the other centres relative to this define the individual sugars, e.g. D-glucose, L-threose, etc. | The configurations of the other centres relative to this define the individual sugars, e.g. D-glucose, L-threose, etc. | ||
| − | [[Image:D-L sugars.png|centre]] | + | [[Image:D-L sugars.png|500px|centre|'''Fischer projections of representative sugars - the configurational "D" or "L" stereogenic centre is denoted with an asterix.''']] |
| + | |||
| + | ==References== | ||
| + | <biblio> | ||
| + | #StickWilliams2009 isbn=9780240521183 | ||
| + | </biblio> | ||
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Latest revision as of 07:46, 17 July 2013
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
The absolute configuration of all monosaccharides is denoted by the configuration at one particular stereocentre in that sugar, namely the stereocentre furthest from the anomeric centre (the carbonyl carbon in the open chain representation) [1]. If, in the Fischer projection, that centre has the hydroxyl group on the right, it is a D-sugar; if on the left, it is an L-sugar. By convention, the "D" and "L" symbols are written in small capitals.
The configurations of the other centres relative to this define the individual sugars, e.g. D-glucose, L-threose, etc.