CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Oxocarbenium ion"
| Line 1: | Line 1: | ||
| − | |||
* Author: [[User:Withers|Stephen Withers]] | * Author: [[User:Withers|Stephen Withers]] | ||
* Responsible Editor: [[User:SpencerWilliams|Spencer Williams]] | * Responsible Editor: [[User:SpencerWilliams|Spencer Williams]] | ||
| Line 6: | Line 5: | ||
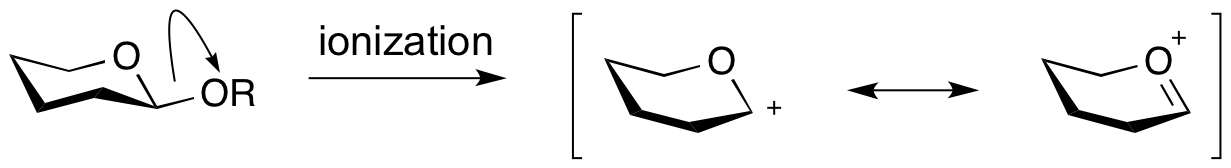
An '''oxocarbenium ion''' is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, they are a positively charged carbocation that is stabilized by resonance. Lifetimes of oxocarbenium ions are estimated at around 10-12 sec: right at the limit of existence as free species. Further, in the presence of anionic nucleophiles their lifetime was shown to be considerably shorter <cite>1</cite>. Therefore it seems unlikely that oxocarbenium ion intermediates exist in the active sites of glycoside hydrolases, which contain nucleophilic carboxylate residues. Indeed there is no experimental evidence for their existence. However, kinetic isotope effect studies have shown that transition states for glycosyl transfer have considerable oxocarbenium ion character. | An '''oxocarbenium ion''' is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, they are a positively charged carbocation that is stabilized by resonance. Lifetimes of oxocarbenium ions are estimated at around 10-12 sec: right at the limit of existence as free species. Further, in the presence of anionic nucleophiles their lifetime was shown to be considerably shorter <cite>1</cite>. Therefore it seems unlikely that oxocarbenium ion intermediates exist in the active sites of glycoside hydrolases, which contain nucleophilic carboxylate residues. Indeed there is no experimental evidence for their existence. However, kinetic isotope effect studies have shown that transition states for glycosyl transfer have considerable oxocarbenium ion character. | ||
| − | [[Image: | + | [[Image:Oxocarbenium.png|centre]] |
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Revision as of 04:16, 11 July 2009
- Author: Stephen Withers
- Responsible Editor: Spencer Williams
An oxocarbenium ion is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, they are a positively charged carbocation that is stabilized by resonance. Lifetimes of oxocarbenium ions are estimated at around 10-12 sec: right at the limit of existence as free species. Further, in the presence of anionic nucleophiles their lifetime was shown to be considerably shorter [1]. Therefore it seems unlikely that oxocarbenium ion intermediates exist in the active sites of glycoside hydrolases, which contain nucleophilic carboxylate residues. Indeed there is no experimental evidence for their existence. However, kinetic isotope effect studies have shown that transition states for glycosyl transfer have considerable oxocarbenium ion character.