CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Carbohydrate Binding Module Family 70"
Menghui Sun (talk | contribs) |
Menghui Sun (talk | contribs) |
||
| Line 23: | Line 23: | ||
== Functionalities == | == Functionalities == | ||
| − | CBM70 domains are commonly found as accessory modules in hyaluronate lyases produced by bacteria of the ''Streptococcus'' genus, such as Hyl from the PL8 family <cite>Daniel2003 Brandi2009</cite>. These domains enhance the enzyme capability to degrade hyaluronic acid, a crucial component of the host's extracellular matrix <cite>Alisdair2004</cite>. Infection by pathogens such as ''S. pneumoniae'' utilize hyaluronate lyase to break down hyaluronic acid, facilitating bacterial invasion and spread <cite>Luciane2002</cite>. CBM70 domains boost this process by increasing the binding efficiency of the enzyme, playing a key role in pathogen virulence and contributing to the high specificity of the enzyme for hyaluronic acid <cite>Kostyukova1995</cite>. Additionally, CBM70 | + | CBM70 domains are commonly found as accessory modules in hyaluronate lyases produced by bacteria of the ''Streptococcus'' genus, such as Hyl from the PL8 family <cite>Daniel2003 Brandi2009</cite>. These domains enhance the enzyme capability to degrade hyaluronic acid, a crucial component of the host's extracellular matrix <cite>Alisdair2004</cite>. Infection by pathogens such as ''S. pneumoniae'' utilize hyaluronate lyase to break down hyaluronic acid, facilitating bacterial invasion and spread <cite>Luciane2002</cite>. CBM70 domains boost this process by increasing the binding efficiency of the enzyme, playing a key role in pathogen virulence and contributing to the high specificity of the enzyme for hyaluronic acid <cite>Kostyukova1995</cite>. Additionally, the CBM70 family member SrCBM70 has been effectively utilized in lateral flow immunoassays for the specific detection of hyaluronic acid, demonstrating its potential in diagnostic applications <cite>Xuanwei2022</cite>. |
== Family Firsts == | == Family Firsts == | ||
Revision as of 19:21, 12 November 2024
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| https://www.cazy.org/CBM70.html |
Ligand specificities
The CBM70 family comprises members predominantly of bacterial origin [1]. Notably, it is the only known family with a specific binding affinity for hyaluronic acid, a linear glycosaminoglycan composed of the repeating disaccharide unit β-1,4-ᴅ-glucuronic acid-β-1,3-N-acetyl-ᴅ-glucosamine. Studies have shown that CBM70 modules typically do not bind to other glycosaminoglycans, such as chondroitin sulfate, dermatan sulfate, or heparin [2].
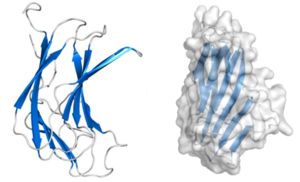
Structural Features
CBM70 modules are typically composed of approximately 160 amino acids. The crystal structure of the N-terminal CBM70 module (SpCBM70) from the Streptococcus pneumoniae hyaluronate lyase Hyl has been determined. SpCBM70 adopts a classic β-jelly roll fold, consisting of two opposing 5-stranded antiparallel β-sheets. This slightly bowed sandwich structure creates a groove along the concave surface, which carries a significant positive charge and is highly conserved within the CBM70 family. This groove, which is similar to the binding site observed in β-sandwich CBMs such as those in the CBM4 family, is the putative hyaluronan-binding site [3]. Structural studies and mutational analysis have identified key residues, including a conserved solvent-exposed tryptophan and several basic residues, that are essential for hyaluronan recognition, supporting its classification as a Type B CBM [1].
Functionalities
CBM70 domains are commonly found as accessory modules in hyaluronate lyases produced by bacteria of the Streptococcus genus, such as Hyl from the PL8 family [4, 5]. These domains enhance the enzyme capability to degrade hyaluronic acid, a crucial component of the host's extracellular matrix [6]. Infection by pathogens such as S. pneumoniae utilize hyaluronate lyase to break down hyaluronic acid, facilitating bacterial invasion and spread [7]. CBM70 domains boost this process by increasing the binding efficiency of the enzyme, playing a key role in pathogen virulence and contributing to the high specificity of the enzyme for hyaluronic acid [8]. Additionally, the CBM70 family member SrCBM70 has been effectively utilized in lateral flow immunoassays for the specific detection of hyaluronic acid, demonstrating its potential in diagnostic applications [2].
Family Firsts
- First Identified
- The first CBM70 module to be identified (SpCBM70) was from the S. pneumoniae hyaluronate lyase Hyl [1].
- First Structural Characterization
- The first crystal structure of a CBM70 module was also that of SpCBM70, PDB ID 4D0Q [1].
References
- Suits MDL, Pluvinage B, Law A, Liu Y, Palma AS, Chai W, Feizi T, and Boraston AB. (2014). Conformational analysis of the Streptococcus pneumoniae hyaluronate lyase and characterization of its hyaluronan-specific carbohydrate-binding module. J Biol Chem. 2014;289(39):27264-27277. DOI:10.1074/jbc.M114.578435 |
- Mei X, Sun M, Zhang Y, Shen J, Li J, Xue C, and Chang Y. (2022). Establishment of a carbohydrate binding module-based lateral flow immunoassay method for identifying hyaluronic acid. Int J Biol Macromol. 2022;223(Pt A):1180-1185. DOI:10.1016/j.ijbiomac.2022.11.122 |
- Boraston AB, Nurizzo D, Notenboom V, Ducros V, Rose DR, Kilburn DG, and Davies GJ. (2002). Differential oligosaccharide recognition by evolutionarily-related beta-1,4 and beta-1,3 glucan-binding modules. J Mol Biol. 2002;319(5):1143-56. DOI:10.1016/S0022-2836(02)00374-1 |
- Rigden DJ and Jedrzejas MJ. (2003). Genome-based identification of a carbohydrate binding module in Streptococcus pneumoniae hyaluronate lyase. Proteins. 2003;52(2):203-11. DOI:10.1002/prot.10405 |
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, and Henrissat B. (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233-8. DOI:10.1093/nar/gkn663 |
- Boraston AB, Bolam DN, Gilbert HJ, and Davies GJ. (2004). Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J. 2004;382(Pt 3):769-81. DOI:10.1042/BJ20040892 |
- Mello LV, De Groot BL, Li S, and Jedrzejas MJ. (2002). Structure and flexibility of Streptococcus agalactiae hyaluronate lyase complex with its substrate. Insights into the mechanism of processive degradation of hyaluronan. J Biol Chem. 2002;277(39):36678-88. DOI:10.1074/jbc.M205140200 |
- Kostyukova NN, Volkova MO, Ivanova VV, and Kvetnaya AS. (1995). A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol Med Microbiol. 1995;10(2):133-7. DOI:10.1111/j.1574-695X.1995.tb00022.x |