CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolase Family 72"
| Line 38: | Line 38: | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | [[File:Gas2FINAL.jpg|thumb|300px|right|'''Figure 1.''' Crystal structure of ''Sc''Gas2 ([{{PDBlink}}2w62 PDB ID 2w62]).]]The first three-dimensional structures available for a GH72 member | + | [[File:Gas2FINAL.jpg|thumb|300px|right|'''Figure 1.''' Crystal structure of ''Sc''Gas2 ([{{PDBlink}}2w62 PDB ID 2w62]).]]The first three-dimensional structures available for a GH72 member were that of ''S. cerevisiae'' ScGas2 in free form ([{{PDBlink}}2w61 PDB ID 2w61]) and in complex with carbohydrates ([{{PDBlink}}2w62 PDB ID 2w62], [{{PDBlink}}2w63 PDB ID 2w63]) (Figure 1). The enzyme folds as a (beta/alpha)<sub>8</sub> barrel similar to that prevailing in other families constituting Clan GH-A <cite>Hurtado-Guerrero2009</cite>. The full length enzyme also harbors a [[CBM43]] module at the C-terminus. The crystal structure also showed that both domains share extensive contacts <cite>Hurtado-Guerrero2009</cite>. |
== Family Firsts == | == Family Firsts == | ||
Revision as of 23:38, 1 January 2016
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Authors: ^^^Ramon Hurtado-Guerrero^^^ and ^^^Thierry Fontaine^^^
- Responsible Curator: ^^^Bernard Henrissat^^^
| Glycoside Hydrolase Family GH72 | |
| Clan | none, (βα)8 fold |
| Mechanism | retaining |
| Active site residues | known |
| CAZy DB link | |
| https://www.cazy.org/GH72.html | |
Substrate specificities
Glycoside hydrolase family GH72 is formed exclusively of transglycosylases of the fungal kingdom. Their activity was first characterized in Aspergillus fumigatus [1] and yeasts [2, 3, 4]. GH72 transglycosidases are GPI-anchored plasma membrane enzymes that elongate and remodel the 1,3-β-glucan of the cell wall [4, 5, 6, 7, 8, 9]. The catalytic domain is located in the external part of the plasma membrane. Two sub-families have been described for GH72 family members depending on the presence or absence of a C-terminal cysteine rich domain (carbohydrate binding domain, CBM43) in addition to the catalytic domain [10].
Kinetics and Mechanism
Catalysis by GH72 family enzymes occurs through a classical Koshland retaining mechanism, which leads to net retention of the β-anomeric configuration of the final product. Enzymatic kinetics were determined by HPLC and showed that these enzymes are transglycosylases rather than glycoside hydrolases. Fungal GH72 enzymes internally cleave a 1,3-β-glucan molecule and form a glycosyl enzyme which reacts with the non-reducing end of a second β-1,3-glucan molecule, forming a new β-1,3-glucosidic linkage, resulting in the truncation of the first chain and elongation of the second. The minimum size of the donor and acceptor substrates so far described are laminaridecaose and laminaripentaose, respectively [1, 11]. Despite the fact that the overall mechanisms of hydrolysis and transglycosylation are well known, it is still unclear how transglycosylases limit or prevent hydrolysis in aqueous media, where the concentration of water is 55 M. By structural studies with different laminarioligosaccharides and enzymatic activity assays, a “base occlusion mechanism”, in which the acceptor sugar blocks the entrance of water molecules, was proposed to explain this phenomenon [12].
Catalytic Residues
Multiple sequence alignments have highlighted conserved amino acid between GH72 family members [13]. Hydrophobic cluster analysis allowed identification of two highly conserved glutamate residues in the region comparable to the C-terminal end of strands β-4 and β-7 of Clostridium cellulolyticum endoglucanase A (a GH5 member) [2]. Site-direct mutagenesis of these two glutamate residues in A. fumigatus Gel1p and S. cerevisiae Gas1p have shown their essentiality for the transglycosidase activity [3, 13] and support the assignment of these residues as the acid-base and nucleophilic residues (Glu-160 and Glu-261, respectively, of Gel1p from C. albicans). The identity of these residues was further confirmed by the resolution of the crystal structure of S. cerevisiae Gas2 (ScGas2) (see below) [12].
Three-dimensional structures
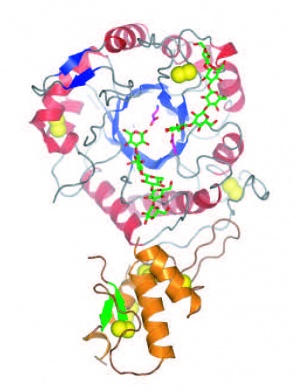
The first three-dimensional structures available for a GH72 member were that of S. cerevisiae ScGas2 in free form (PDB ID 2w61) and in complex with carbohydrates (PDB ID 2w62, PDB ID 2w63) (Figure 1). The enzyme folds as a (beta/alpha)8 barrel similar to that prevailing in other families constituting Clan GH-A [12]. The full length enzyme also harbors a CBM43 module at the C-terminus. The crystal structure also showed that both domains share extensive contacts [12].
Family Firsts
- First stereochemistry determination
β-1,3-glucanosyltransglycosylase (Gel1p) from Aspergillus fumigatus [1]
- First catalytic nucleophile identification
Shown in the β-1,3-glucanosyltransglycosylase (Gel1p) from Aspergillus fumigatus [13]
- First general acid/base residue identification
Shown in the β-1,3-glucanosyltransglycosylase (Gel1p) from Aspergillus fumigatus [13]
- First 3-D structure
- ScGas2 crystal structure [12]
References
- Hartland RP, Fontaine T, Debeaupuis JP, Simenel C, Delepierre M, and Latgé JP. (1996). A novel beta-(1-3)-glucanosyltransferase from the cell wall of Aspergillus fumigatus. J Biol Chem. 1996;271(43):26843-9. DOI:10.1074/jbc.271.43.26843 |
- Mouyna I, Fontaine T, Vai M, Monod M, Fonzi WA, Diaquin M, Popolo L, Hartland RP, and Latgé JP. (2000). Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J Biol Chem. 2000;275(20):14882-9. DOI:10.1074/jbc.275.20.14882 |
- Carotti C, Ragni E, Palomares O, Fontaine T, Tedeschi G, Rodríguez R, Latgé JP, Vai M, and Popolo L. (2004). Characterization of recombinant forms of the yeast Gas1 protein and identification of residues essential for glucanosyltransferase activity and folding. Eur J Biochem. 2004;271(18):3635-45. DOI:10.1111/j.1432-1033.2004.04297.x |
- de Medina-Redondo M, Arnáiz-Pita Y, Fontaine T, Del Rey F, Latgé JP, and Vázquez de Aldana CR. (2008). The beta-1,3-glucanosyltransferase gas4p is essential for ascospore wall maturation and spore viability in Schizosaccharomyces pombe. Mol Microbiol. 2008;68(5):1283-99. DOI:10.1111/j.1365-2958.2008.06233.x |
- Mouyna I, Fontaine T, Vai M, Monod M, Fonzi WA, Diaquin M, Popolo L, Hartland RP, and Latgé JP. (2000). Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J Biol Chem. 2000;275(20):14882-9. DOI:10.1074/jbc.275.20.14882 |
- Mouyna I, Morelle W, Vai M, Monod M, Léchenne B, Fontaine T, Beauvais A, Sarfati J, Prévost MC, Henry C, and Latgé JP. (2005). Deletion of GEL2 encoding for a beta(1-3)glucanosyltransferase affects morphogenesis and virulence in Aspergillus fumigatus. Mol Microbiol. 2005;56(6):1675-88. DOI:10.1111/j.1365-2958.2005.04654.x |
- Gastebois A, Fontaine T, Latgé JP, and Mouyna I. (2010). beta(1-3)Glucanosyltransferase Gel4p is essential for Aspergillus fumigatus. Eukaryot Cell. 2010;9(8):1294-8. DOI:10.1128/EC.00107-10 |
- de Medina-Redondo M, Arnáiz-Pita Y, Clavaud C, Fontaine T, del Rey F, Latgé JP, and Vázquez de Aldana CR. (2010). β(1,3)-glucanosyl-transferase activity is essential for cell wall integrity and viability of Schizosaccharomyces pombe. PLoS One. 2010;5(11):e14046. DOI:10.1371/journal.pone.0014046 |
- Ragni E, Coluccio A, Rolli E, Rodriguez-Peña JM, Colasante G, Arroyo J, Neiman AM, and Popolo L. (2007). GAS2 and GAS4, a pair of developmentally regulated genes required for spore wall assembly in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6(2):302-16. DOI:10.1128/EC.00321-06 |
- Ragni E, Fontaine T, Gissi C, Latgè JP, and Popolo L. (2007). The Gas family of proteins of Saccharomyces cerevisiae: characterization and evolutionary analysis. Yeast. 2007;24(4):297-308. DOI:10.1002/yea.1473 |
- Mazáň M, Ragni E, Popolo L, and Farkaš V. (2011). Catalytic properties of the Gas family β-(1,3)-glucanosyltransferases active in fungal cell-wall biogenesis as determined by a novel fluorescent assay. Biochem J. 2011;438(2):275-82. DOI:10.1042/BJ20110405 |
- Hurtado-Guerrero R, Schüttelkopf AW, Mouyna I, Ibrahim AF, Shepherd S, Fontaine T, Latgé JP, and van Aalten DM. (2009). Molecular mechanisms of yeast cell wall glucan remodeling. J Biol Chem. 2009;284(13):8461-9. DOI:10.1074/jbc.M807990200 |
- Mouyna I, Monod M, Fontaine T, Henrissat B, Léchenne B, and Latgé JP. (2000). Identification of the catalytic residues of the first family of beta(1-3)glucanosyltransferases identified in fungi. Biochem J. 2000;347 Pt 3(Pt 3):741-7. | Google Books | Open Library