CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolase Family 186"
Sei Motouchi (talk | contribs) |
Sei Motouchi (talk | contribs) |
||
| Line 36: | Line 36: | ||
General acid and base of EcOpgD are D388 and D300, respectively<cite>MotouchiEc2023</cite>. | General acid and base of EcOpgD are D388 and D300, respectively<cite>MotouchiEc2023</cite>. | ||
== Three-dimensional structures == | == Three-dimensional structures == | ||
| − | The ligand-free structure of OpgG from ''E. coli'' (EcOpgG) was determined at 2.5 Å (PDB: 1txk)<cite>Hanoulle2004</cite>. The ligand-free structure of EcOpgD was determined at 2.95 Å (PDB: 8IOX)<cite>MotouchiEc2023</cite>. Michaelis complexes of EcOpgD (D388N, co-crystal) and EcOpgG (D361N, soaking) with β-1,2-glucan were determined at 2.06, 1.81 Å, respectively (PDB: 8IP1, 8IP2)<cite>MotouchiEc2023</cite>. [[File:The overall structure of Michaelis complex of EcOpgG (monomer).jpg|thumb]]There is no structural homolog of GH186 in whole GH families. EcOpgG consists of an N-terminal domain (residues 22–388, β-sandwich) and a C-terminal domain (residues 401–511, Ig-like fold). The two domains are connected with one turn of 3<sub>10</sub> helix<cite>Hanoulle2004 MotouchiEc2023</cite>. The loop region (residues 409-425, Loop A below) in the C-terminal domain of the ligand-free structure changes into β-strands in the Michaelis complex structure. In the Michaelis complex, the β-strands reach for the catalytic center of another chain in the dimer to cover the proton transfer pathway from a nucleophile to the general base catalyst<cite>MotouchiEc2023</cite>. However, the sequence of Loop A is diversified in GH186 family. Indeed, Loop A in EcOpgD sequesters the proton transfer pathway from the solvent, while that of EcOpgG does not completely, which is consistent with the drastically reduced hydrolytic activity of EcOpgG compared with EcOpgD<cite>MotouchiEc2023</cite>. | + | The ligand-free structure of OpgG from ''E. coli'' (EcOpgG) was determined at 2.5 Å (PDB: 1txk)<cite>Hanoulle2004</cite>. The ligand-free structure of EcOpgD was determined at 2.95 Å (PDB: 8IOX)<cite>MotouchiEc2023</cite>. Michaelis complexes of EcOpgD (D388N, co-crystal) and EcOpgG (D361N, soaking) with β-1,2-glucan were determined at 2.06, 1.81 Å, respectively (PDB: 8IP1, 8IP2)<cite>MotouchiEc2023</cite>. [[File:The overall structure of Michaelis complex of EcOpgG (monomer).jpg|thumb]]There is no structural homolog of GH186 in whole GH families<cite>MotouchiEc2023</cite>. EcOpgG consists of an N-terminal domain (residues 22–388, β-sandwich) and a C-terminal domain (residues 401–511, Ig-like fold). The two domains are connected with one turn of 3<sub>10</sub> helix<cite>Hanoulle2004 MotouchiEc2023</cite>. The loop region (residues 409-425, Loop A below) in the C-terminal domain of the ligand-free structure changes into β-strands in the Michaelis complex structure. In the Michaelis complex, the β-strands reach for the catalytic center of another chain in the dimer to cover the proton transfer pathway from a nucleophile to the general base catalyst<cite>MotouchiEc2023</cite>. However, the sequence of Loop A is diversified in GH186 family. Indeed, Loop A in EcOpgD sequesters the proton transfer pathway from the solvent, while that of EcOpgG does not completely, which is consistent with the drastically reduced hydrolytic activity of EcOpgG compared with EcOpgD<cite>MotouchiEc2023</cite>. |
== Family Firsts == | == Family Firsts == | ||
;First stereochemistry determination: EcOpgD by optical rotation<cite>MotouchiEc2023</cite>. | ;First stereochemistry determination: EcOpgD by optical rotation<cite>MotouchiEc2023</cite>. | ||
Revision as of 01:30, 25 January 2024
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
| Glycoside Hydrolase Family GH186 | |
| Clan | GH-x |
| Mechanism | inverting |
| Active site residues | Asp |
| CAZy DB link | |
| https://www.cazy.org/GH186.html | |
Substrate specificities
The defining member of GH186, a β-1,2-glucanase from Escherichia coli (EcOpgD) was identified, characterized and structurally analyzed as reported in 2023[1].EcOpgD is specific toward β-1,2-glucan and the amino acid residues for recognizing β-1,2-glucan are highly conserved in GH186[1]. EcOpgD preferentially generate β-1,2-glucooligosaccharides (Sopns, n is degree of polymerization, DP) with DPs of 6 and 7 from linear β-1,2-glucan[1]. Final products produced by EcOpgD are Sop6–10, indicating that EcOgpD hydrolyzes Sopns with DPs of 11 and higher[1]. Almost all family members are found in Pseudomonadota, especially in gamma proteobacteria. Functionally important residues in EcOpgD are not conserved in most of GH186 homologs[1].
Kinetics and Mechanism
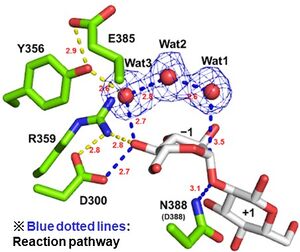
Optical rotation analysis indicates that EcOpgD adopt anomer-inverting hydrolytic mechanism[1]. X-ray structural analysis and mutational analysis suggest that D388 in EcOpgD directly protonates the scissile glycoside bond as general acid[1]. These analyses also suggest that D300 in EcOpgD activates the nucleophilic water via 4-hydroxy group of the Glc moiety at subsite –1 and two water molecules as general base[1]. Thus, EcOpgD has unique long proton transfer pathway from nucleophilic water to general base.
Catalytic Residues
General acid and base of EcOpgD are D388 and D300, respectively[1].
Three-dimensional structures
The ligand-free structure of OpgG from E. coli (EcOpgG) was determined at 2.5 Å (PDB: 1txk)[2]. The ligand-free structure of EcOpgD was determined at 2.95 Å (PDB: 8IOX)[1]. Michaelis complexes of EcOpgD (D388N, co-crystal) and EcOpgG (D361N, soaking) with β-1,2-glucan were determined at 2.06, 1.81 Å, respectively (PDB: 8IP1, 8IP2)[1].
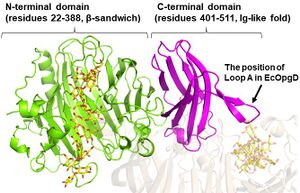
There is no structural homolog of GH186 in whole GH families[1]. EcOpgG consists of an N-terminal domain (residues 22–388, β-sandwich) and a C-terminal domain (residues 401–511, Ig-like fold). The two domains are connected with one turn of 310 helix[1, 2]. The loop region (residues 409-425, Loop A below) in the C-terminal domain of the ligand-free structure changes into β-strands in the Michaelis complex structure. In the Michaelis complex, the β-strands reach for the catalytic center of another chain in the dimer to cover the proton transfer pathway from a nucleophile to the general base catalyst[1]. However, the sequence of Loop A is diversified in GH186 family. Indeed, Loop A in EcOpgD sequesters the proton transfer pathway from the solvent, while that of EcOpgG does not completely, which is consistent with the drastically reduced hydrolytic activity of EcOpgG compared with EcOpgD[1].
Family Firsts
- First stereochemistry determination
- EcOpgD by optical rotation[1].
- First general acid residue identification
- EcOpgD by X-ray crystallography and site-directed mutagenesis[1].
- First general base residue identification
- EcOpgD by X-ray crystallography and site-directed mutagenesis[1].
- First 3-D structure
- EcOpgG by X-ray crystallography[2].
References
- Motouchi S, Kobayashi K, Nakai H, and Nakajima M. (2023). Identification of enzymatic functions of osmo-regulated periplasmic glucan biosynthesis proteins from Escherichia coli reveals a novel glycoside hydrolase family. Commun Biol. 2023;6(1):961. DOI:10.1038/s42003-023-05336-6 |
- Hanoulle X, Rollet E, Clantin B, Landrieu I, Odberg-Ferragut C, Lippens G, Bohin JP, and Villeret V. (2004). Structural analysis of Escherichia coli OpgG, a protein required for the biosynthesis of osmoregulated periplasmic glucans. J Mol Biol. 2004;342(1):195-205. DOI:10.1016/j.jmb.2004.07.004 |