CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Catalytic nucleophile"
(Created page with 'The term '''catalytic nucleophile''' refers to an amino acid residue in a glycoside hydrolase. The residue participates in the classical Koshland retaining mechanism. In …') |
|||
| Line 1: | Line 1: | ||
| + | * Author: [[User:SpencerWilliams|Spencer Williams]] | ||
| + | * Responsible Curator: [[User:SpencerWilliams|Spencer Williams]] | ||
| + | ---- | ||
| + | == Overview == | ||
| + | |||
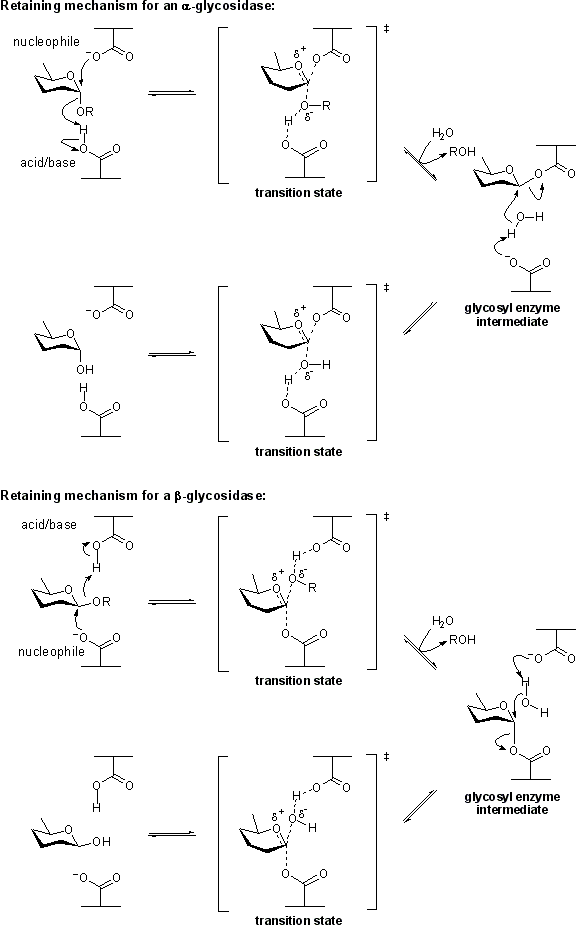
The term '''catalytic nucleophile''' refers to an amino acid residue in a [[glycoside hydrolase]]. The residue participates in the [[classical Koshland retaining mechanism]]. In summary, hydrolysis occurs with net retention of configuration through a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. The reaction occurs with acid/base assistance provided by another amino acid side chain. In the first step (often called the glycosylation step), the catalytic nucleophile plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a base catalyst deprotonating the water molecule as it attacks. At the completion of the catalytic cycle the catalytic nucleophile is restored. | The term '''catalytic nucleophile''' refers to an amino acid residue in a [[glycoside hydrolase]]. The residue participates in the [[classical Koshland retaining mechanism]]. In summary, hydrolysis occurs with net retention of configuration through a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. The reaction occurs with acid/base assistance provided by another amino acid side chain. In the first step (often called the glycosylation step), the catalytic nucleophile plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a base catalyst deprotonating the water molecule as it attacks. At the completion of the catalytic cycle the catalytic nucleophile is restored. | ||
[[Image:Retaining_glycosidase_mechanism_1.png|centre]] | [[Image:Retaining_glycosidase_mechanism_1.png|centre]] | ||
| − | + | ==Methods for indentifying the catalytic nucleophile== | |
| + | |||
| + | == References == | ||
| + | <biblio> | ||
| + | |||
| + | </biblio> | ||
| + | [[Category:Definitions and explanations]] | ||
Revision as of 00:18, 30 August 2009
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
Overview
The term catalytic nucleophile refers to an amino acid residue in a glycoside hydrolase. The residue participates in the classical Koshland retaining mechanism. In summary, hydrolysis occurs with net retention of configuration through a two step, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. The reaction occurs with acid/base assistance provided by another amino acid side chain. In the first step (often called the glycosylation step), the catalytic nucleophile plays the role of a nucleophile, attacking the anomeric centre to displace the aglycon and form a glycosyl enzyme intermediate. At the same time the other residue functions as an acid catalyst and protonates the glycosidic oxygen as the bond cleaves. In the second step (known as the deglycosylation step), the glycosyl enzyme is hydrolyzed by water, with the other residue now acting as a base catalyst deprotonating the water molecule as it attacks. At the completion of the catalytic cycle the catalytic nucleophile is restored.