CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Oxazolinium ion"
| Line 7: | Line 7: | ||
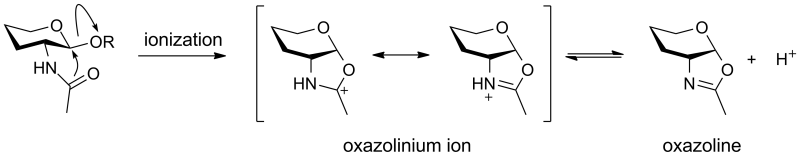
An '''oxazolinium ion''' is the species that is formed by anchimeric assistence in the glycosidic bond-cleaving reaction of a 2-deoxy-2-acetamido glycoside <cite>Tews1997</cite>. Solution studies have shown that the 2-acetamido ''N''-acetyl group enhances spontaneous hydrolysis, of methyl 2-acetamido-2-deoxy-α-D-glucopyranoside relative to methyl α-D-glucopyranoside, by more than 1000-fold <cite>Piszkeiwicz1968</cite>. An oxazolinium ion can be deprotonated to give an oxazoline, which is a stable and isolable species. Oxazolinium ions are common intermediates in the [[neighboring group participation]] mechanism of [[glycoside hydrolase]]s, including glycoside hydrolases from families [[GH18]], [[GH20]], [[GH25]], [[GH56]], [[GH84]], and [[GH85]]. | An '''oxazolinium ion''' is the species that is formed by anchimeric assistence in the glycosidic bond-cleaving reaction of a 2-deoxy-2-acetamido glycoside <cite>Tews1997</cite>. Solution studies have shown that the 2-acetamido ''N''-acetyl group enhances spontaneous hydrolysis, of methyl 2-acetamido-2-deoxy-α-D-glucopyranoside relative to methyl α-D-glucopyranoside, by more than 1000-fold <cite>Piszkeiwicz1968</cite>. An oxazolinium ion can be deprotonated to give an oxazoline, which is a stable and isolable species. Oxazolinium ions are common intermediates in the [[neighboring group participation]] mechanism of [[glycoside hydrolase]]s, including glycoside hydrolases from families [[GH18]], [[GH20]], [[GH25]], [[GH56]], [[GH84]], and [[GH85]]. | ||
| − | [[Image:oxazolinium_ion.png|thumb|centre|800px| | + | [[Image:oxazolinium_ion.png|thumb|centre|800px|''Mechanism for conversion of a 2-acetamido-2-deoxy-β-D-glucopyranoside to an oxazolinium ion and oxazoline.'']] |
Anchimeric assistance can occur with modified 2-acetamido groups, such as 2-glycolylamido groups, affording hydroxymethyl substituted oxazolinium ion intermediates, such as that demonstrated for the glycoside hydrolases of [[GH84]] <cite>Macauley2012</cite>. | Anchimeric assistance can occur with modified 2-acetamido groups, such as 2-glycolylamido groups, affording hydroxymethyl substituted oxazolinium ion intermediates, such as that demonstrated for the glycoside hydrolases of [[GH84]] <cite>Macauley2012</cite>. | ||
Revision as of 19:54, 6 February 2013
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Spencer Williams
- Responsible Curator: Spencer Williams
An oxazolinium ion is the species that is formed by anchimeric assistence in the glycosidic bond-cleaving reaction of a 2-deoxy-2-acetamido glycoside [1]. Solution studies have shown that the 2-acetamido N-acetyl group enhances spontaneous hydrolysis, of methyl 2-acetamido-2-deoxy-α-D-glucopyranoside relative to methyl α-D-glucopyranoside, by more than 1000-fold [2]. An oxazolinium ion can be deprotonated to give an oxazoline, which is a stable and isolable species. Oxazolinium ions are common intermediates in the neighboring group participation mechanism of glycoside hydrolases, including glycoside hydrolases from families GH18, GH20, GH25, GH56, GH84, and GH85.
Anchimeric assistance can occur with modified 2-acetamido groups, such as 2-glycolylamido groups, affording hydroxymethyl substituted oxazolinium ion intermediates, such as that demonstrated for the glycoside hydrolases of GH84 [3].
See also
References
-
Tews, I., Terwisscha van Scheltinga, A.C., Perrakis, A., Wilson, K.S., and Dijkstra, B.W. J. Am. Chem. Soc. 1997, 119, 7954-7959.
-
Piszkiewicz, D.; Bruice, T. C. J. Am. Chem. Soc. 1968, 90, 5844-5848.
- Macauley MS, Chan J, Zandberg WF, He Y, Whitworth GE, Stubbs KA, Yuzwa SA, Bennet AJ, Varki A, Davies GJ, and Vocadlo DJ. (2012). Metabolism of vertebrate amino sugars with N-glycolyl groups: intracellular β-O-linked N-glycolylglucosamine (GlcNGc), UDP-GlcNGc, and the biochemical and structural rationale for the substrate tolerance of β-O-linked β-N-acetylglucosaminidase. J Biol Chem. 2012;287(34):28882-97. DOI:10.1074/jbc.M112.363721 |