CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Auxiliary Activity Family 5
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Maria Cleveland^^^ and ^^^Yann Mathieu^^^
- Responsible Curator: ^^^Harry Brumer^^^
| Auxiliary Activity Family AA5 | |
| Fold | Seven-bladed β-propeller |
| Mechanism | Copper Radical Oxidase |
| Active site residues | known |
| CAZy DB link | |
| https://www.cazy.org/AA5.html | |
Substrate Specificities
Enzymes from Auxiliary Activity Family 5 (AA5) are mononuclear copper-radical oxidases (CROs) that perform the two-electron oxidation of substrates using oxygen as the final electron acceptor (EC 1.1.3.-) [1]. AA5 members are further classified into two major subfamilies [2]: Subfamily AA5_1 contains characterized glyoxal oxidases (EC 1.2.3.15) [3]. Subfamily AA5_2 contains galactose 6-oxidases (EC 1.1.3.9), which oxidize the C-6 hydroxyl of diverse galactosides to the corresponding aldehyde [4, 5, 6]. AA5_2 also contains the more recently discovered general alcohol oxidases (EC 1.1.3.13) [6, 7, 8] and aryl alcohol oxidases (EC 1.1.3.7) [9, 10]. The first biochemically characterized member of AA5 was the galactose 6-oxidase from the phytopathogenic fungus Fusarium graminearum (previously known as Polyporus circinatus and Cladobotryum (Dactylium) dendroides [11]), which was originally reported in 1959 following isolation from cultures [12, 13]. Subsequently, the Fusarium graminearum galactose 6-oxidase became the defining member of AA5_2 [2]. The first characterized member of what is now known as AA5_1 is the glyoxal oxidase from Phanerochaete chrysosporium, which was likewise isolated from fungal culture [14].
In contrast to their fungal and bacterial counterparts, plant AA5 members do not fall within the two defined subfamilies. An AA5 enzyme from Arabidopsis thaliana has been demonstrated in vivo to have galactose 6-oxidase activity and promote cell-to-cell adhesion in the seed coat epidermis [15] (see also [16]. Additionally, a Streptomyces lividans enzyme, GlxA, which is distantly related to AA5, has been shown to oxidize glycolaldehyde and a deletion mutant showed a loss of glycan accumulation at hyphal tips [17].
AA5_1
The AA5_1 members are generally known as glyoxal oxidases (EC 1.2.3.15), characterized members of which typically accept a range of simple aldehydes, α-hydroxycarbonyl, and α-dicarbonyl compounds as substrates, with the highest activities observed on glyoxal, methylglyoxal and glycolaldehyde [1, 14, 18, 19, 20]. In contrast, two glyoxal oxidases form Pycnoporus cinnabarinus demonstrated the highest catalytic efficiency on glyoxylic acid [21]. An apparent distinction between the AA5_1 and AA5_2 subfamilies is that while AA5_1 enzymes catalyze the oxidation of aldehydes to carboxylic acids [1], AA5_2 members oxidize primary alcohols to the corresponding aldehyde (and, in some instances, also oxidize the aldehyde to the acid, albeit much more slowly) [4, 22]. Consequently, the oxidation of aldehydes by AA5 CROs has been suggested to proceed through the hydrated, gem-diol species [18].
AA5_2
The archetypal CRO and AA5 member is the Fusarium graminearum galactose 6-oxidase (FgrGalOx), which catalyzes the regioselective oxidation of the C6-hydroxyl group on the monosaccharide galactose (EC 1.3.3.7) [12, 13]. The range of substrates oxidized by FgrGalOx also includes galactosides as methyl beta-galactopyranoside [23], and galactose-containing di-, oligo-, and polysaccharides, including lactose, melibiose, raffinose, galactoxyloglucan, galactomannan and galactoglucomannan [22, 24]. Several other AA5_2 members from Fusarium species, such as those from F. oxysporum [25], F. sambucinum [23], and F. acuminatum [26] have substrate specificities similar to FgrGalOx. The last distinct group of AA5_2 enzymes based on substrate specificity are the raffinose-specific oxidases (EC 1.3.3.-). Other AA5_2 orthologs exhibit specificity for the alpha-galactosyl unit of the di- and trisaccharides mellibiose and raffinose, respectively, over galactose [5, 6]. For many decades since their discovery, galactose 6-oxidase activity was thought to be the defining feature of this family, although a limited ability of FgrGalOx to oxidize non-carbohydrate alcohols has been noted [27, 28]
In 2015, two AA5_2 orthologs from the fungi Colletotrichum graminicola and Colletotrichum gloeosporioides were characterized (CgrAlcOx and CglAlcOx, respectively), which were essentially inactive on galactose and galactosides, but efficiently oxidized the hydroxyl group of diverse aliphatic and aromatic primary alcohols [7]. These enzymes exhibited high catalytic efficiency towards, e.g., n-butan-1-ol, 2,4-hexadiene-1-ol, benzyl alcohol, and cinnamyl alcohol, and were therefore denoted as general alcohol oxidases (EC 1.3.3.13) [7]. Likewise, two AA5_2 members were characterized from the pathogenic fungi Pyricularia oryzae (PorAlcOx) and Colletotrichum higginsianum (ChiAlcOx), which exhibited prominent activity on n-butan-1-ol, ethanol, 1,3-butanediol, and glycerol [8]. Since then, additional AA5_2 enzymes from various fungi have been characterized as general alcohol oxidases, some of which efficiently oxidize both carbohydrate and non-carbohydrate substrates [6]. More specifically, several fungal AA5_2 members, including homologs from Colletotrichum/Glomerella and Fusarium species, have been characterized as aryl alcohol oxidases due to predominant specificities toward substituted benzyl alcohols and 5-hydroxymethylfurfural (HMF) (see EC 1.1.3.7 and EC 1.1.3.47) [6, 9, 10].
The specificity of AA5 CROs has been harnessed for a range of biotechnological applications. The earliest examples include glycoprotein labelling via oxidation of galactosyl residues with FgrGalOx [ADD REFS]. Likewise, FgrGalOx has been utilized for the production of functionalized carbohydrates from biomass sources [29, 30, 31, 32, 33, 34, 35, 36]. The ability of specific AA5 members to oxidize aliphatic and aromatic alcohols to the corresponding aldehydes, including stereoselectively, has biocatalytic applications in chemical production, e.g. for the pharmaceutical and food and fragrance industries [ADD REFS, including coupled reaction examples] [31, 37, 38, 39]. Similarly, the ability of CROs to convert HMF into the bi-functional precursors diformylfuran (DFF) and 2,5-furandicarboxylic acid (FDCA) may find application in polymer manufacturing [9] [ADD REFS]. Several variants of FgrGalOx have been developed to enable such applications [ADD REFS].
Kinetics and Mechanism
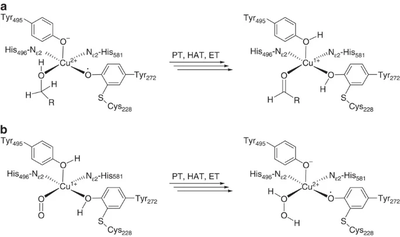
The majority of what is known about the mechanism of AA5 enzymes comes from studies on the archetype, FgrGalOx. AA5 enzymes oxidize their substrates through a ping-pong mechanism involving a corresponding reduction of oxygen to hydrogen peroxide mediated by a mononuclear copper center, which is complexed via a distinct, crosslinked tyrosyl-cysteine residue (see below) [1, 4, 18, 19, 40, 41, 42, 43]. The first half-reaction results in a two-electron oxidation of the substrate and corresponding reduction of the Cu[II]-tyrosyl radical to a Cu[I]-tyrosine (phenol). The second half-reaction regenerates the oxidation state of the active-site through reduction of molecular oxygen to hydrogen peroxide. Detailed kinetic studies, including kinetic isotope effects, suggest that each half reaction consists of three steps: proton transfer (PT), hydrogen atom transfer (HAT), and electron transfer (ET) [42, 44]. Due to its fundamental uniqueness, the mechanism of AA5 CROs has received significant theoretical treatment and the synthesis of many chemical mimetics has been attempted [45, 46].
Practically, AA5 enzymes are conveniently assayed by measuring hydrogen peroxide (co-product) generatation, e.g. in coupled reactions with horseradish peroxidase and a chromogenic substrate. In preparative reactions, catalase is typically added to prevent accumulation of hydrogen peroxide. AA5 enzymes are prone to inactivation by one-electron reduction to a Cu[I]-tyrosyl radical. The resulting off-cycle species can be rescued by oxidation by peroxidases or transition metal ions (ferricyanide, Mg(III), etc.), the inclusion of which in reactions is required to obtain maximal activity and substrate conversion [39, 47, 48, 49, 50, 51, 52].
Catalytic Residues
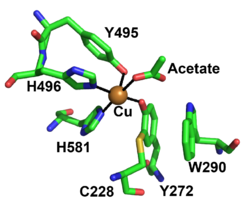
The redox-active center of AA5 oxidases comprises a copper ion that coordinated by two tyrosine sidechains and two histidine sidechains (in FgrGalOx these are Tyr495, Tyr272, His496, and His581, respectively), resulting in a distorted square pyramidal geometry [7, 9, 53, 54]. Based on the copper coordination environment, AA5 proteins are type 2 "non-blue" copper enzymes due to the nitrogen and oxygen ligands [54]. The unique feature of AA5 enzymes is the covalently linked equatorial tyrosine with an adjacent cysteine by a thioether bond (Tyr272 and Cys228 in the archetypal FgrGalOx) [53]. The thioether linkage forms spontaneously in the presence of copper and has been shown to stabilize the radical though delocalization onto the equatorial tyrosine during catalysis [55].
Another important feature of AA5 enzymes is a secondary shell amino acid that is located on top of the tyrosine-cysteine cofactor (Trp290 in FgrGalOx). It has been speculated to be critical in determining the substrate specificity, radical stability and redox activity by hydrogen bonding, delocalization and/or by protecting the thioether bond from solvent [1, 3, 4, 56]. This residue in AA5_1, based on sequence alignments, has been conserved as a histidine [1], while characterized AA5_2 enzymes have an aromatic residue at this position: a tryptophan in galactose oxidases (W290 in FgrGalOx) [6, 56], a phenylalanine in the Colletotrichum aliphatic alcohol oxidases [7], whereas a tyrosine is present in the raffinose oxidases [5, 6] and aryl alcohol oxidase from Colletotrichum graminicola [9]. Furthermore, an AA5 enzyme from Streptomyces lividans with activity on glycolaldehyde possesses a tryptophan as the stacking secondary shell residue whose indole ring is oriented differently compared to FgrGalOx, which may affect the substrate specificity [17, 57].
In AA5_2 the Tyr-Cys cofactor exhibits an unusually low reduction potential (+275 mV) [58, 59, 60] compared to unmodified tyrosine in solution (> +800 mV) or in other enzymatic systems [61]. Several factors could contribute to this phenomenon by increasing the stability of the protein free radical including π-stacking with aromatic residues and the electron donating effect of the thioether linkage [4, 56, 62]. In contrast, AA5_1 have a reduction potential around +640 mV [18] which could explain the different oxidizing power of these two subfamilies [1, 60]. One reason for the higher reduction potential of glyoxal oxidases could be subtitution of the secondary shell amino acid Trp in AA5_2 with a His in AA5_1 [1, 60]. In the archetypal AA5_2 member, FgrGalOx, the Trp290His substitution increased the reduction potential of the resulting enzyme from +400 mV to +730 mV [63]; however, it also decreased the catalytic efficiency by 1000-fold [41] and affected the stability of the [Cu2+ Tyr·] metallo-radical complex at neutral pH [64]. CgrAlcOx and CgrAAO have been speculated to have a lower reduction potential than FgrGalOx due to their secondary shell amino acid substitutions (Phe in CgrAlcOx and Tyr in CgrAAO) [7, 9].
Three-dimensional Structures
AA5 members share a core seven-bladed β-propeller fold containing the active site (Figure 3) [7, 9, 54]. The structure of the archetype, FgrGalOx, was first reported in 1991 and comprises three domains: Domain 1 has a beta-sandwich structure now known as Carbohydrate Binding Module Family 32, Domain 2 is the catalytic domain, and Domain 3 is a small, β-strand domain that packs against the catalytic domain on the side opposite from the active-site, stabilizing domain 2 [53, 54]. Particularly notably, the original structural analysis of FgrGalOx revealed the distinct crosslinked Tyr-Cys active site residue of CROs, provided the first CBM32 tertiary structure, and indicated the ability of Domain 1/CBM32 to bind galactose [53].
The CBM32 domain is widely, but not exclusively, conserved among many AA5_2 members, especially from Fusarium species [6, 23, 25, 65] (including some that do not posses predominant galactose 6-oxidase activity, e.g. FgrAAO and FoxAAO [6, 10].) In other cases, PAN and WSC domains are found in place of the CBM32. The function of PAN domains in Colletotrichum graminicola aryl alchohol oxidase and raffinose oxidase is unclear [5, 9], while the WSC domain in Pyricularia oryzae alchohol oxidase was able to bind xylans and fungal chitin/β-1,3-glucan, implicating the involvement of this domain in enzyme anchoring [8]. Finally, several general alcohol oxidases (i.e. those with little activity toward galactosides) do not possess a corresponding N-terminal domain, but rather comprise only the seven-bladed β-propeller and C-terminal domain (Figure 3) [7, 8]
Family Firsts
- First AA5_1 enzyme discovered
- The glyoxal oxidase from Phanerochaete chrysosporium discovered in 1987 [14].
- First AA5_2 enzyme discovered
- The archetypal galactose-6 oxidase from Fusarium graminearum (FgrGalOx) discovered in 1959 [12].
- Copper requirement confirmed
- While this first report already established FgrGalOx as a metalloenzyme; its copper requirement was later confirmed [66].
- First 3-D structure
- The first crystallography structure of AA5 was of the archetypal FgrGalOx in 1991 [53].
References
Error fetching PMID 13863403:
Error fetching PMID 26680532:
Error fetching PMID 15504031:
Error fetching PMID 30885320:
Error fetching PMID 3553159:
Error fetching PMID 13641238:
Error fetching PMID 14012475:
Error fetching PMID 34134727:
Error fetching PMID 24967652:
Error fetching PMID 25543085:
Error fetching PMID 31177409:
Error fetching PMID 8182749:
Error fetching PMID 26858983:
Error fetching PMID 28390013:
Error fetching PMID 2002850:
Error fetching PMID 27626829:
Error fetching PMID 12203454:
Error fetching PMID 8386015:
Error fetching PMID 11551381:
Error fetching PMID 12797833:
Error fetching PMID 9622494:
Error fetching PMID 17385891:
Error fetching PMID 8557673:
Error fetching PMID 24915038:
Error fetching PMID 7929198:
Error fetching PMID 10593910:
Error fetching PMID 15581579:
Error fetching PMID 19290629:
Error fetching PMID 9438841:
Error fetching PMID 164209:
Error fetching PMID 183480:
Error fetching PMID 32108208:
Error fetching PMID 33533375:
Error fetching PMID 26205496:
Error fetching PMID 28093470:
Error fetching PMID 30852555:
Error fetching PMID 34042158:
Error fetching PMID 3791303:
Error fetching PMID 24188837:
Error fetching PMID 22422625:
Error fetching PMID 22724576:
Error fetching PMID 26892369:
Error fetching PMID 17518413:
Error fetching PMID 20000571:
Error fetching PMID 34613753:
Error fetching PMID 34147589:
Error fetching PMID 27260365:
Error fetching PMID 15578222:
Error fetching PMID 11607073:
Error fetching PMID 18771294:
Error fetching PMID 22226201:
- Error fetching PMID 24915038:
- Levasseur A, Drula E, Lombard V, Coutinho PM, and Henrissat B. (2013). Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels. 2013;6(1):41. DOI:10.1186/1754-6834-6-41 |
- Error fetching PMID 28390013:
- Error fetching PMID 12797833:
- Error fetching PMID 28778886:
-
pmid=
- Error fetching PMID 26680532:
- Error fetching PMID 30885320:
-
Mathieu, Y., Offen, W. A., Forget, S. M., Ciano, L., Viborg, A. H., Blagova, E., Henrissat, B., Walton, P.H, Davies, G.J, and Brumer, H. (2020). Discovery of a fungal copper radical oxidase with high catalytic efficiency toward 5-hydroxymethylfurfural and benzyl alcohols for bioprocessing. ACS Catalysis, 10, 3042-3058. DOI:10.1021/acscatal.9b04727
- Error fetching PMID 34134727:
-
Ögel, Z. B.; Brayford, D.; McPherson, M. J., (1994). Cellulose-triggered sporulation in the galactose oxidase-producing fungus Cladobotryum (Dactylium) dendroides NRRL 2903 and its re-identification as a species of Fusarium. Mycol. Res., 98, 474-480. DOI:10.1016/S0953-7562(09)81207-0
- Error fetching PMID 13641238:
- Error fetching PMID 13863403:
- Error fetching PMID 3553159:
- Error fetching PMID 30852555:
- Error fetching PMID 34042158:
- Error fetching PMID 26205496:
- Error fetching PMID 8557673:
- Error fetching PMID 10593910:
- Error fetching PMID 15578222:
- Error fetching PMID 27260365:
-
Parikka, K.; Master, E.; Tenkanen, M., (2015) Oxidation with galactose oxidase: Multifunctional enzymatic catalysis. J. Mol. Catal. B: Enzym. 120, 47-59. https://doi.org/10.1016/j.molcatb.2015.06.006
- Error fetching PMID 25543085:
- Error fetching PMID 20000571:
- Error fetching PMID 24967652:
- Error fetching PMID 17518413:
- Error fetching PMID 15504031:
-
Siebum, A., van Wijk, A., Schoevaart, R. & Kieboom, T. (2006) Galactose oxidase and alcohol oxidase: scope and limitations for the enzymatic synthesis of aldehydes. J. Mol. Catal. B, 41, 141–145. DOI:10.1016/j.molcatb.2006.04.003
-
Yalpani, M.; Hall, L. D., (1982) Some chemical and analytical aspects of polysaccharide modifications. II. A high-yielding, specific method for the chemical derivatization of galactose-containing polysaccharides: Oxidation with galactose oxidase followed by reductive amination. J. Polym. Sci., Polym. Chem. Ed. 20, 3399-3420. https://doi.org/10.1002/pol.1982.170201213
- Error fetching PMID 3791303:
-
Schoevaart, R., Kieboom, T. (2004) Application of galactose oxidase in chemoenzymatic one-pot cascade reactions without intermediate recovery steps. Topics in Catalysis, 27, 3–9. DOI:10.1023/B:TOCA.0000013536.27551.13
-
Schoevaart, R.; Kieboom, T., (2004) Application of Galactose Oxidase in Chemoenzymatic One-Pot Cascade Reactions Without Intermediate Recovery Steps. Top. Catal. 27, 3-9. https://doi.org/10.1023/B:TOCA.0000013536.27551.13
- Error fetching PMID 24188837:
- Error fetching PMID 22422625:
- Error fetching PMID 22724576:
-
Mikkonen, K. S.; Parikka, K.; Suuronen, J.-P.; Ghafar, A.; Serimaa, R.; Tenkanen, M., (2014) Enzymatic oxidation as a potential new route to produce polysaccharide aerogels. RSC Advances. 4, 11884-11892. https://doi.org/10.1039/C3RA47440B
- Error fetching PMID 26892369:
- Error fetching PMID 34613753:
- Error fetching PMID 34147589:
- Error fetching PMID 22226201:
- Error fetching PMID 15581579:
- Error fetching PMID 7929198:
- Error fetching PMID 19290629:
- Error fetching PMID 8386015:
- Error fetching PMID 9622494:
- Error fetching PMID 9438841:
-
Himo, F.; Eriksson, L. A.; Maseras, F.; Siegbahn, P. E. M., (2000). Catalytic Mechanism of Galactose Oxidase: A Theoretical Study. J. Am. Chem. Soc. 122, 8031-8036. https://doi.org/10.1021/ja994527r
- Error fetching PMID 11607073:
- Error fetching PMID 164209:
- Error fetching PMID 183480:
-
Toftgaard Pedersen, A.; Birmingham, W. R.; Rehn, G.; Charnock, S. J.; Turner, N. J.; Woodley, J. M., (2015) Process Requirements of Galactose Oxidase Catalyzed Oxidation of Alcohols. Org. Process Res. Dev. 19, 1580-1589. https://doi.org/10.1021/acs.oprd.5b00278
- Error fetching PMID 32108208:
- Error fetching PMID 33533375:
- Error fetching PMID 2002850:
- Error fetching PMID 8182749:
- Error fetching PMID 18771294:
- Error fetching PMID 17385891:
- Error fetching PMID 28093470:
- Error fetching PMID 27626829:
- Error fetching PMID 12203454:
- Error fetching PMID 11551381:
-
Itoh, S.; Taki, M.; Fukuzumi, S., (2000). Active site models for galactose oxidase and related enzymes. Coord. Chem. Rev. 198, 3-20. https://doi.org/10.1016/S0010-8545(99)00209-X
-
Jazdzewski, B. A.; Tolman, W. B., (2000). Understanding the copper–phenoxyl radical array in galactose oxidase: contributions from synthetic modeling studies. Coord. Chem. Rev. 200-202, 633-685. https://doi.org/10.1016/S0010-8545(00)00342-8
-
Saysell, C. G.; Barna, T.; Borman, C. D.; Baron, A. J.; McPherson, M. J.; Sykes, A. G., P(1997). Properties of the Trp290His variant of Fusarium NRRL 2903 galactose oxidase: interactions of the GOasesemi state with different buffers, its redox activity and ability to bind azide. J. Biol. Inorg. Chem. 2, 702-709. https://doi.org/10.1007/s007750050186
-
Rogers, M. S.; Knowles, P. F.; Baron, A. J.; McPherson, M. J.; Dooley, D. M., (1998). Characterization of the active site of galactose oxidase and its active site mutational variants Y495F/H/K and W290H by circular dichroism spectroscopy. Inorg. Chim. Acta. 275-276, 175-181. https://doi.org/10.1016/S0020-1693(97)06142-2
- Error fetching PMID 31177409:
- Error fetching PMID 14012475:
- Error fetching PMID 26858983:
