CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
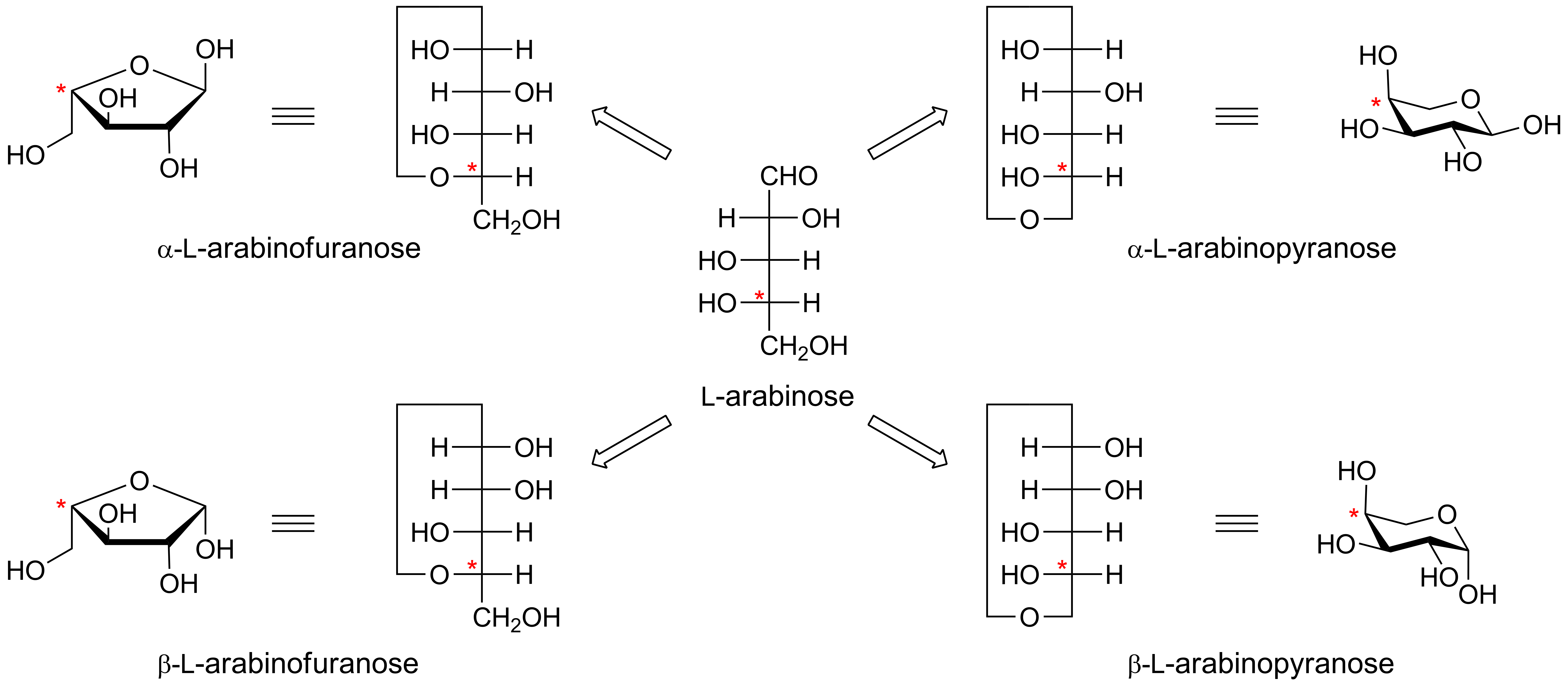
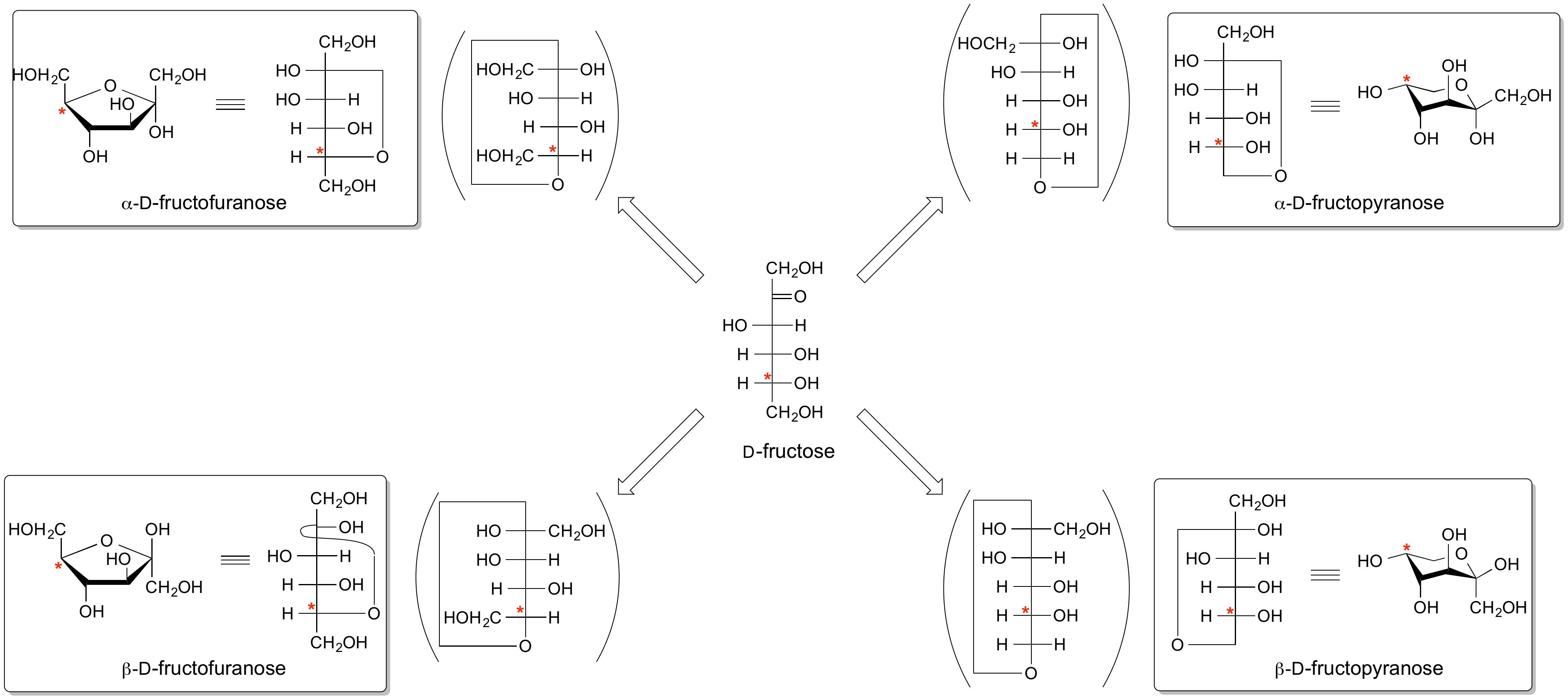
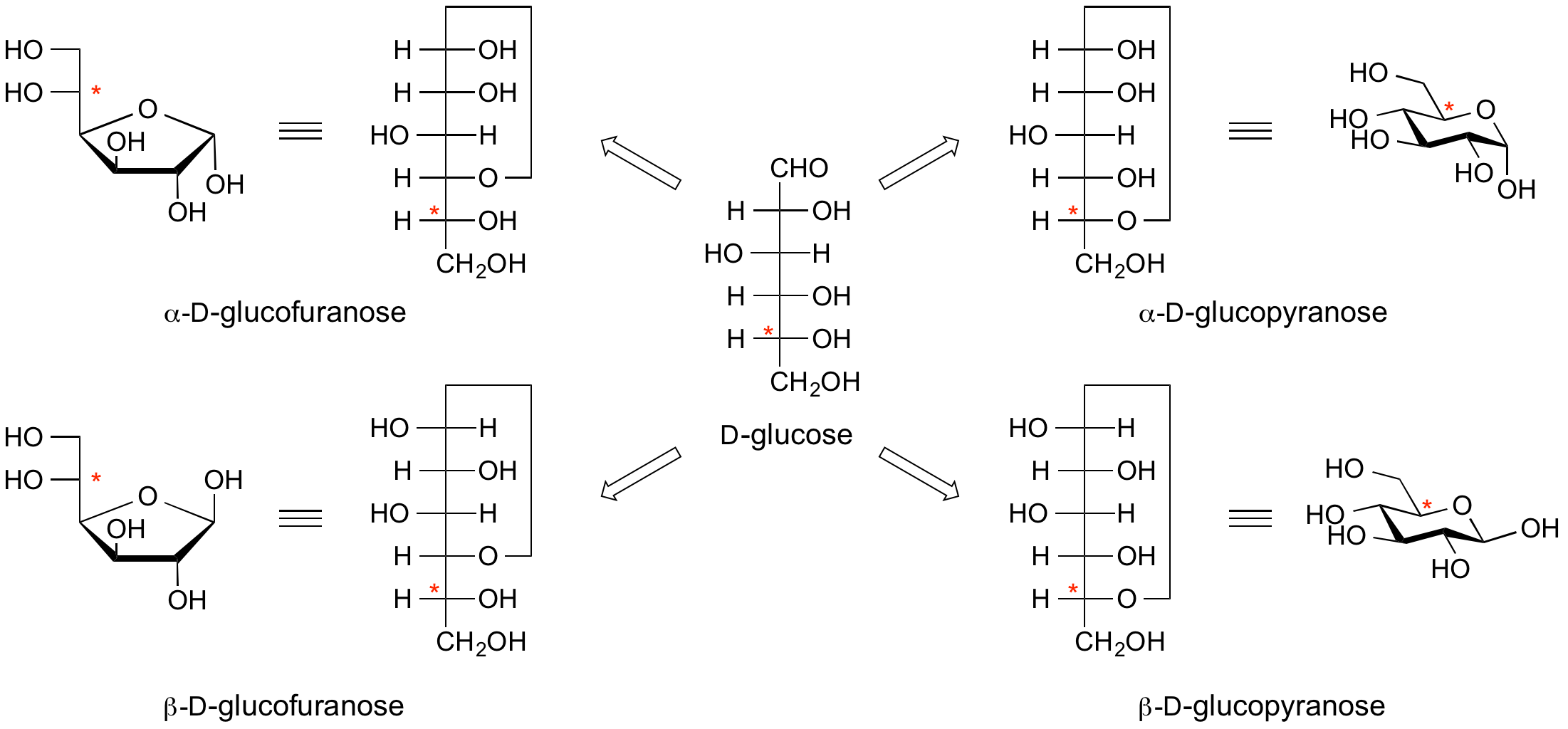
Anomeric centre (alpha and beta)
The anomeric centre of a sugar is a stereocentre created from the intramolecular formation of an acetal (or ketal) of a sugar hydroxyl group and an aldehyde (or ketone) group. The two stereoisomers formed from the two possible stereochemistries at the anomeric centre are called anomers. These are diastereoisomers of one another.
The configuration at the anomeric centre (that derived from the carbonyl carbon) is denoted alpha- or beta- by reference to the stereocentre that determines the absolute configuration. In a Fischer projection, if the substituent off the anomeric centre is on the same side as the oxygen of the configurational (D- or L-) carbon, then it is the alpha-anomer. If it is directed in the opposite direction it is the beta-anomer.
In the case of D-hexoses drawn in the 'usual' Haworth projection, alpha-anomer is the one with the anomeric substituent on the opposite face to the C5 (hydroxymethyl) substitutent, ie directed ‘down’; the beta-anomer is that with the anomeric substituent being on the same face as the C5 hydroxymethyl substitutent, ie directed up.
References
- Carbohydrates: The essential molecules of life, R.V. Stick, S.J. Williams, Elsevier, 2009, 474 pages.