CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Glycoside Hydrolase Family 102
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
- Author: ^^^Anthony Clarke^^^
- Responsible Curator: ^^^Anthony Clarke^^^
| Glycoside Hydrolase Family GHnn | |
| Clan | GH-x |
| Mechanism | retaining/inverting |
| Active site residues | known/not known |
| CAZy DB link | |
| http://www.cazy.org/fam/GHnn.html | |
Substrate specificities
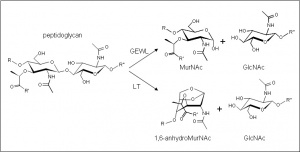
The glycoside hydrolases of this family are lytic transglyosylases (also referred to as peptidoglycan lyases) of bacterial origin and they constitute family 2 of the classification scheme of Blackburn and Clarke [1]. The prototype for this family is membrane-bound lytic transglycosylase A (MltA) from Escherichia coli. These enzymes cleave the β-1,4 linkage between N-acetylmuramoyl and N-acetylglucosaminyl residues in peptidoglycan (Figure 1), but unlike the lytic transglycosylases of other families, they are active on peptidoglycan fragments lacking their stem peptides [2]. No other activities have been observed.
Kinetics and Mechanism
The lytic transglycosidases, strictly speaking, are retaining enzymes. However, they are not hydrolases but rather catalyse an intramolecular glycosyl transferase reaction onto the C-6 hydroxyl group of the muramoyl residue leading to the generation of a terminal 1,6-anhdyromuramoyl product thus lacking a reducing end [3]. No detailed analyses involving both steady state and pre-steady state kinetic studies have been reported.
Catalytic Residues
Content is to be added here.
Three-dimensional structures
Content is to be added here.
Family Firsts
- First sterochemistry determination
- Cite some reference here, with a short (1-2 sentence) explanation [4].
- First catalytic nucleophile identification
- Cite some reference here, with a short (1-2 sentence) explanation [5].
- First general acid/base residue identification
- Cite some reference here, with a short (1-2 sentence) explanation [6].
- First 3-D structure
- Cite some reference here, with a short (1-2 sentence) explanation [3].
References
- Ursinus A and Höltje JV. (1994). Purification and properties of a membrane-bound lytic transglycosylase from Escherichia coli. J Bacteriol. 1994;176(2):338-43. DOI:10.1128/jb.176.2.338-343.1994 |
[[Category:Glycoside Hydrolase Families|GH102]