CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Glycoside Hydrolase Family 174
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
| Glycoside Hydrolase Family GH174 | |
| Clan | GH-x |
| Mechanism | retaining/inverting |
| Active site residues | known/not known |
| CAZy DB link | |
| https://www.cazy.org/GH174.html | |
Substrate specificities
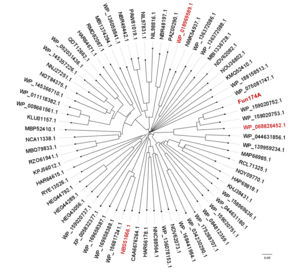
Members of glycoside hydrolase family 174 have been shown to exhibit α-1,3-L-fucanase activity. The first member of this family, Fun174A from a marine bacterium Wenyingzhuangia aestuarii OF219, specifically hydrolyze the α-1,3- L-fucoside bonds between 2-O-sulfated and non-sulfated fucose residues in the sulfated fucan from sea cucumber Isostichopus badionotus in a processive endo-acting manner.[1] Meanwhile, three homologs of Fun174A from Rubritalea marina, Spartobacteria bacterium and Wenyingzhuangia fucanilytica, display activities toward sulfated fucan from Isostichopus badionotus.[2] All 92 members (up to May,2023) of GH174 are bacterial enzymes.
Kinetics and Mechanism
The catalytic mechanism of GH174 has not been identified. As mentioned in the report, Fun174A showed no transglycosylating activity in the tested acceptor substrates, such as glycerin, methanol, L-fucose, D-glucose, D-galactose, D-fructose, D-mannose, D-glucosamine and N-acetyl- D-glucosamine.[3]
Catalytic Residues
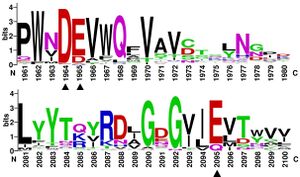
Multiple sequence alignments of GH174 homologs showed that D119, E120 and E218 in Fun174A were highly conserved in all sequences. Three single-site mutants D119E, E120A and E218Q were established, expressed and identified in the report. Mutant D119E, E120A and E218Q resulted in 100.0%, 85.7% and 88.3% loss of activity on sulfated fucan from Isostichopus badionotus respectively. It indicated that D119, E120 and E218 were critical for the functioning of Fun174A.[4]
Three-dimensional structures
No three-dimensional structure has been solved in this glycoside hydrolase family at present.
Family Firsts
- First stereochemistry determination
- Not yet identified.
- First catalytic nucleophile identification
- Not yet identified.
- First general acid/base residue identification
- Not yet identified.
- First 3-D structure
- Not yet identified.