CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Carbohydrate Binding Module Family 14
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| CAZy DB link | |
| https://www.cazy.org/CBM14.html |
Ligand specificities
Family 14 CBMs are modules composed of approximately 70 residues. These modules have been reported to be associated with chitinases [1] and as chitin-binding lectins e.g. effector proteins from the tomato pathogens Pseudoercospora fuligena or Cladosporium fulvum [2, 3], as an antimicrobial protein (tachycitin) from horseshoe crab (Tachypleus tridentatus) hemocytes [4] and in peritrophic matrix proteins from the malaria vector Anopheles gambia [5]. Members of CBM14 have been shown to bind chitin [5, 6, 7] and chitooligomers [3, 8]; binding to 50 % acetylated hyaluronan has also been demonstrated [8].
The ligand binding affinities have been quantified for two CBM14 members. The interaction between a CBM14 from human chitotriosidase-1 (CHIT1, characterized as a glycoside hydrolase family 18 (GH18)) and (GlcNAc)3 has been investigated. The CBM14 displayed a relatively weak interaction of KD 9.9 ± 0.8 mM using NMR titration experiments [8] and KD 3.1 ± 0.2 mM using isothermal titration calorimetry (ITC) [7].
Interaction studies have also been performed for a CBM14 from the fungal tomato pathogen C. fulvum and (GlcNAc)6. Here, the binding properties were measured using ITC yielding a KD of 6.7 ± 1.5 µM [3].
Structural Features
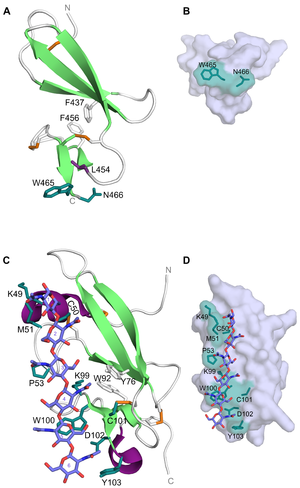
CBM14 members have a hevein-like fold made up by a central β-sheet (three anti-parallel β-strands) linked to a small β-sheet (two anti-parallel β-strands) by two aromatic residues. The CBM14 members from tomato pathogens also have an N-terminal α-helix and an extended loop connecting -strands 2 and 3, as well as a C-terminal helical turn [2, 3]. The latter is also present in tachycitin [9]. In addtion, the CBM14 structures have been reported to contain 3-4 disulfide bridges which also serve as a stabilizing effect on this unique fold [1, 3, 9]. With the hevein-like fold and lectin-like properties, members of the CBM14 family are characterized as type C CBMs [10]. This is in line with the ligand-binding feature seen for the CBM14 associated with CHIT1 (Homo sapiens CBM14, HsCBM14). The binding site is composed of a tryptophan and an asparagine (Trp465 and Asn466) at the C-terminus of the module [8] (Figure 1A). These residues create a platform-like binding surface (Figure 1B) commonly seen in type A CBMs and could be the reason for why HsCBM14 is also able to bind crystalline chitin. In addition to Trp465 and Asn466, a leucine (Leu454) has been reported to be indirectly involved in binding as it contributes to maintaining a correct orientation for Trp465 [7]. Binding between HsCBM14 and chitotriose is likely to occur through CH-π stacking between the side-chain of Trp465 and the middle pyranose ring and hydrogen bonds between the side-chain of Asn466 and the hydrophilic non-reducing end [7].
CBM14 from the tomato pathogen C. fulvum (CfCBM14) was the first of these modules to be co-crystallized with (GlcNAc)6 (PDB ID: 6BN0)[3]. Although the fold of CfCBM14 is the same as HsCBM14 (PDB ID: 5HBF and 6SO0) and other structurally characterized CBM14 members, the residues responsible for binding differ. Binding of (GlcNAc)6 is mediated by Lys49, Cys50, Met51, Pro53, Lys99, Trp100, Cys101, Asp102 and Tyr103 positioned along a shallow trench in the longitudinal axis of CfCBM14 (Figure 1C and D). Mutational studies showed that binding to (GlcNAc)6 was abolished by mutation of Trp100 and Asp102. The main interaction is reported to be a CH-π stacking between Trp100 and GlcNAc-5 aided by Met51 and Pro53 which are both in van der Waal distances to GlcNAc-1 and GlcNAc-3, respectively [3]. Although equivalents to Trp100, Asp102 and Tyr103 have been reported to be important for chitin-binding in CBM14 from tomato pathogen P. fuligena (PfCBM14) (equivalent residues: Trp94, Asp96 and Tyr97) [2], the extensive hydrogen bond network could point to a different binding mechanism for CfCBM14 that is more reminiscent of a type B CBM.
Functionalities
Several members of CBM14 are often encountered as chitin-binding lectins [2, 3, 4, 9, 11], while the members present in modular chitinases play a key role in targeting substrate [1, 12, 13]. The most common associated modules are chitinases, e.g. GH18 from human chitotriosidase structurally determined together with HsCBM14 (PDB ID: 5HBF)[1].
Although some of the structural features described above differ between the CBM14 lectins and HsCBM14, the common denominator is the involvement of these modules in immune responses [1, 14, 15]. CHIT1 is produced by macrophages and neutrophils [16, 17] and is utilized by the innate immune response to combat chitin-containing pathogens [18]. A different response is found in the tomato pathogen C. fulvum; here, the effector protein utilizes CBM14's ability to bind chitin in the fungal cell wall enabling protection from hydrolysis by plant-derived chitinases during infection [14, 15].
Family Firsts
- First Identified
- CBM14 was first identified as an antimicrobial protein with chitin-binding ability in the hemocyte of horseshoe crab (T. tridentatus) and called tachycitin [4].
- First Structural Characterization
- The first three-dimensional structure was determined by NMR spectroscopy for the CBM14 module tachycitin (PDB ID: 1DQC) [9]. The structure of the first carbohydrate-active enzyme associated CBM14 was determined by X-ray crystallography for the CBM14 of human chitotriosidase (PDB ID: 5HBF) [1].
References
Error fetching PMID 27401545:
Error fetching PMID 30148881:
Error fetching PMID 9010778:
Error fetching PMID 9651363:
Error fetching PMID 21674664:
Error fetching PMID 31891077:
Error fetching PMID 28584264:
Error fetching PMID 10770921:
Error fetching PMID 14769793:
Error fetching PMID 9118991:
Error fetching PMID 10617646:
Error fetching PMID 8132768:
Error fetching PMID 19662198:
Error fetching PMID 19169854:
Error fetching PMID 9090881:
Error fetching PMID 17153926:
- Error fetching PMID 27111557:
- Error fetching PMID 27401545:
- Error fetching PMID 30148881:
- Error fetching PMID 9010778:
- Error fetching PMID 9651363:
- Error fetching PMID 21674664:
- Error fetching PMID 31891077:
- Error fetching PMID 28584264:
- Error fetching PMID 10770921:
- Boraston AB, Bolam DN, Gilbert HJ, and Davies GJ. (2004). Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J. 2004;382(Pt 3):769-81. DOI:10.1042/BJ20040892 |
- Error fetching PMID 14769793:
- Error fetching PMID 9118991:
- Error fetching PMID 10617646:
- Error fetching PMID 17153926:
- Error fetching PMID 9090881:
- Error fetching PMID 8132768:
- Error fetching PMID 19662198:
- Error fetching PMID 19169854: