CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Conformational nomenclature
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
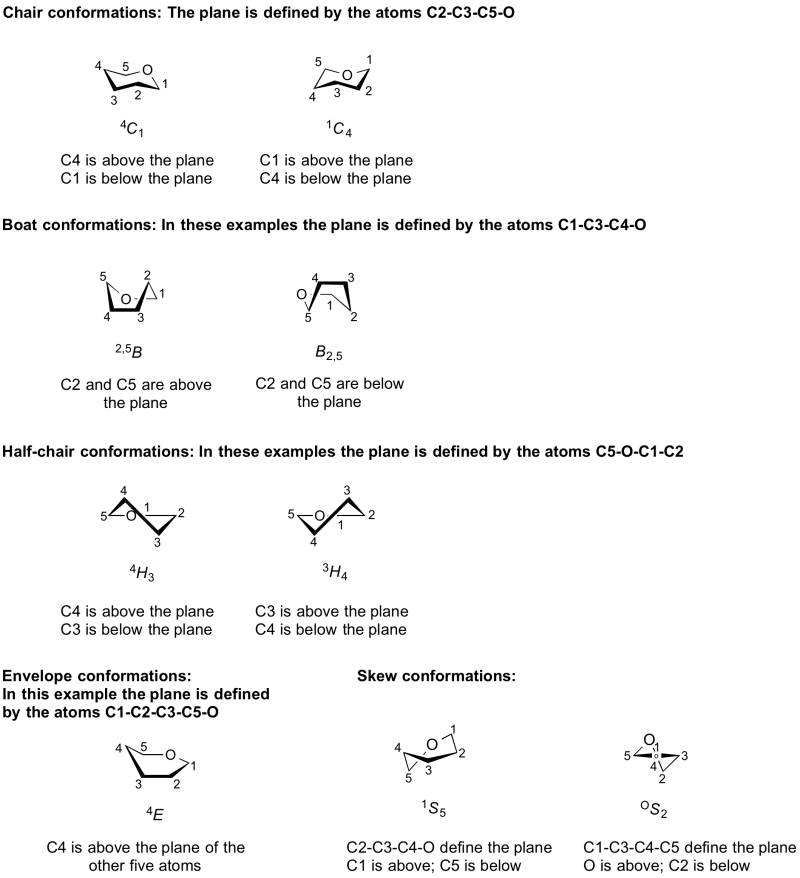
The conformations adopted by a pyranose or furanose ring are denoted by a system in which a capital letter indicates the overall shape, C = chair, B = boat, H = half chair, S = skew boat, E = envelope [1, 2]. The first four of these conformations has four atoms in a plane; the envelope conformation has five.
The particular conformation is then denoted by assigning the letter corresponding to the shape (C, B, H, S, E); determining the four (or five) atoms that define the plane; assigning a 'top' and 'bottom' face through the use of a left-hand rule counting in the order of increasing ring carbon; and then indicating the identities and relative positions (top face = superscript and prefix; bottom face = subscript and suffix) of the remaining two atoms on that capital letter. In the case of the envelope conformation, only a single atom is located outside of the plane. For a more detailed discussion see the excellent book by J. Fraser Stoddard [3].
It should be noted that the conformational symbols for enantiomers are different. This is because the reference plane is the same, yet application of the left-hand rule results in a different 'top' and 'bottom' face. Thus the mirror image of α-D-glucose-4C1 is α-L-glucose-1C4. It is therefore important to state whether the D or L form is under consideration.
References
-
Conformational nomenclature for five- and six-membered ring forms of monosaccharides and their derivatives, Pure and Appl. Chem., 1981, 53, 1901—1905. DOI: 10.1351/pac198153101901
- (1980). IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Conformational nomenclature for five and six-membered ring forms of monosaccharides and their derivatives: recommendations 1980. Eur J Biochem. 1980;111(2):295-8. DOI:10.1111/j.1432-1033.1980.tb04941.x |
-
Stereochemistry of Carbohydrates, J. Fraser Stoddart, John Wiley & Sons Inc, 1971, 264 pages.