CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Glycoside Hydrolase Family 19
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| Glycoside Hydrolase Family GH19 | |
| Clan | none (lysozyme fold) |
| Mechanism | inverting |
| Active site residues | known |
| CAZy DB link | |
| https://www.cazy.org/GH19.html | |
Substrate specificities
Glycoside hydrolases of family 19 are endo-acting enzymes that hydrolyze glycosidic bonds within chitin, an insoluble polymer of β-1,4-linked N-acetyl-D-glucosamine (GlcNAc) and are thus referred to as chitinases (EC 3.2.1.14). These enzymes were originally identified in plants. In an older classification system for plant chitinases, comprising both GH18 and GH19 chitinases, family 19 enzymes comprise classes I, II and IV. In 1996, the first bacterial family 19 chitinase was described [1]. In addition to cleaving chitin, GH19 chitinases cleave soluble oligomers of β-1,4-linked N-acetyl-D-glucosamine. For some plant enzymes lysozyme activity has been demonstrated. Currently available data suggest that GH19 enzymes are not particularly effective in degrading crystalline chitin (compared to certain members of the GH18 chitinase family), especially enzymes that lack CBMs. On the other hand GH19 enzymes are highly active on chitosans (= partially deacetylated chitin) with high degrees of acetylation, even if they lack a CBM [2, 3]. Detailed studies on GH19 chitinases from Streptomyces (class IV; [3]) and rice (Oryza sativa; Class I; [4]) have revealed that productive binding requires a GlcNAc to be bound in subsites -2 and +1, whereas deacetylated GlcNAc (GlcN) is tolerated in subsites -1 and +2.
Kinetics and Mechanism
Family 19 enzymes employ an inverting mechanism, as determined by NMR [5] and HPLC analysis[6]. Both structural characteristics (see below) and available biochemical data [3, 4] suggest that GH19 chitinases are non-processive endo-acting enzymes. Kinetic data for the conversion of polymeric and oligomeric substrates have been described in several studies. In some studies, kinetic data have been used to derive subsite binding affinties (e.g. [7, 8]).
Catalytic Residues

The catalytic residues are two glutamates. Although there still is limited structural information underpinning details of the inverting catalytic mechanism, there is considerable support for the notion that a glutamate located at the end of the third alpha helix (Glu 67 in the barley enzyme) acts as the catalytic general acid, whereas another glutamate located in a more variable loop-like structure (Glu89 in the barley enzyme) acts as the catalytic general base[9, 11, 12, 13].
It has been shown that at least two more conserved charged residues are crucial for catalysis. These residues, Glu203 and Arg215 in barley chitinase, form a triad together with the catalytic acid Glu67 [14] (see Figure). Interestingly, a similarly complex electrostatic interaction network is present in family GH46 chitosanases [15, 16] with whom the family 19 enzymes share some overall structural similarity (see below).
Three-dimensional structures
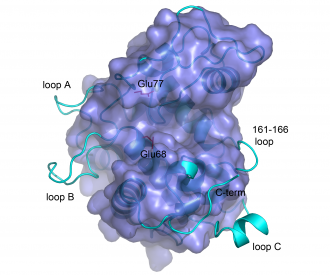
The catalytic domains of family 19 chitinases have a lysozyme-like fold with rather shallow substrate-binding grooves that are not particularly rich in aromatic residues (see Figure). The catalytic domains of family 19 chitinases share a common fold with family GH46 chitosanases and with lysozymes in families GH22, GH23 and GH24 of glycoside hydrolases [17]. For a long time, structural information for these chitinases was limited to the structures of two class II plant enzymes [11, 18]. Recently, the structures of bacterial family GH19 chitinases have become available ([13, 19]; class IV), as well as the structures of class I [20] and class IV [21] GH19 chitinases from plants.
The structures of bacterial GH19 chitinases revealed several differences from the previously reported plant structures ([13, 19]; see Figure). Compared to plant enzymes, the bacterial enzymes lack a C-terminal extension and three loops, some of which are thought to be flexible [20, 22].
There is no structural information for GH19 enzymes in complex with their substrate. In 2008, Huet et al published [9] the structure of a complex of papaya family 19 chitinase with GlcNAc units bound in the -2 and +1 subsites. This structure has been used to build a plausible model of a complex with (GlcNAc)4. This is the first structure (half experimental, half modeled) of an enzyme-substrate complex.
Structure images

Family Firsts
- First primary sequence determination
- Bean leaf chitinase [23]
- First general base residue identification
- Chitinase from barley; determination by site-directed mutagenesis [12], structural analysis [11] and modelling [24]. Additional support from structure determination and modelling of a papaya chitinase [9].
- First general acid residue identification
- Chitinase from barley; determination by site-directed mutagenesis [12], structural analysis [11] and modelling [24]. Additional support from structure determination and modelling of a papaya chitinase [9].
- First 3-D structure
- Barley chitinase [11].
References
Error fetching PMID 16636468:
Error fetching PMID 19222164:
Error fetching PMID 16957091:
Error fetching PMID 8612749:
Error fetching PMID 9774706:
Error fetching PMID 12825688:
Error fetching PMID 8421299:
Error fetching PMID 9148754:
Error fetching PMID 17010167:
Error fetching PMID 16272567:
Error fetching PMID 10829022:
Error fetching PMID 10957628:
Error fetching PMID 17608716:
Error fetching PMID 19629717:
Error fetching PMID 19332152:
Error fetching PMID 2428042:
Error fetching PMID 9539727:
- Error fetching PMID 8752320:
- Error fetching PMID 16636468:
- Error fetching PMID 19222164:
- Error fetching PMID 16957091:
-
Fukamizo T, Koga D, Goto S Comparative biochemistry of chitinases - anomeric form of the reaction products Bioscience, Biotechnology & Biochemistry 1995, 59:311-313.
- Error fetching PMID 8612749:
- Error fetching PMID 9774706:
- Error fetching PMID 12825688:
- Huet J, Rucktooa P, Clantin B, Azarkan M, Looze Y, Villeret V, and Wintjens R. (2008). X-ray structure of papaya chitinase reveals the substrate binding mode of glycosyl hydrolase family 19 chitinases. Biochemistry. 2008;47(32):8283-91. DOI:10.1021/bi800655u |
-
Hoell IA, Vaaje-Kolstad G, Eijsink VGH Structure and function of enzymes acting on chitin and chitosan Biotechnology and Genetic Engineering Reviews, Vol. 27, 2010, in press.
- Error fetching PMID 8421299:
- Error fetching PMID 9148754:
- Error fetching PMID 17010167:
- Error fetching PMID 16272567:
- Error fetching PMID 10829022:
- Lacombe-Harvey ME, Fukamizo T, Gagnon J, Ghinet MG, Dennhart N, Letzel T, and Brzezinski R. (2009). Accessory active site residues of Streptomyces sp. N174 chitosanase: variations on a common theme in the lysozyme superfamily. FEBS J. 2009;276(3):857-69. DOI:10.1111/j.1742-4658.2008.06830.x |
- Monzingo AF, Marcotte EM, Hart PJ, and Robertus JD. (1996). Chitinases, chitosanases, and lysozymes can be divided into procaryotic and eucaryotic families sharing a conserved core. Nat Struct Biol. 1996;3(2):133-40. DOI:10.1038/nsb0296-133 |
- Error fetching PMID 10957628:
- Kezuka Y, Ohishi M, Itoh Y, Watanabe J, Mitsutomi M, Watanabe T, and Nonaka T. (2006). Structural studies of a two-domain chitinase from Streptomyces griseus HUT6037. J Mol Biol. 2006;358(2):472-84. DOI:10.1016/j.jmb.2006.02.013 |
- Error fetching PMID 17608716:
- Error fetching PMID 19629717:
- Error fetching PMID 19332152:
- Error fetching PMID 2428042:
- Error fetching PMID 9539727: