CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Oxocarbenium ion"
| (24 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{CuratorApproved}} | ||
* Author: [[User:Withers|Stephen Withers]] | * Author: [[User:Withers|Stephen Withers]] | ||
| − | * Responsible | + | * Responsible Curator: [[User:SpencerWilliams|Spencer Williams]] |
---- | ---- | ||
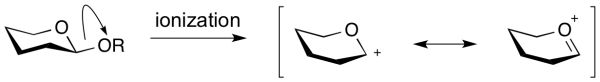
| − | An '''oxocarbenium ion''' is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, | + | An '''oxocarbenium ion''' is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, it is a positively charged carbocation that is stabilized by resonance. |
| + | == Lifetime and involvement in enzymatic mechanisms == | ||
| + | Lifetimes of oxocarbenium ions in water are estimated at around 10<sup>-12</sup> sec: right at the limit of existence as free species. Further, in the presence of anionic nucleophiles their lifetime was shown to be considerably shorter <cite>Banait1991</cite>. Therefore it seems unlikely that oxocarbenium ion intermediates exist in the active sites of [[glycoside hydrolases]], which contain nucleophilic carboxylate residues. Indeed there is no experimental evidence for their existence. However, kinetic isotope effect studies have shown that [[transition state]]s for glycosyl transfer have considerable oxocarbenium ion character. | ||
| − | [[Image:Oxocarbenium.png| | + | [[Image:Oxocarbenium.png|center|600px]] |
| − | + | For [[glycosyltransferases]] the situation is less clear, and there remains the possibility that an oxocarbenium ion may represent a bonafide reaction intermediate. For this to be the case the active site must effectively exclude water and must not contain nucleophilic residues in close proximity to the reactive centre. | |
| − | [[Image:Oxocarbenium_conformations.png| | + | == Conformation == |
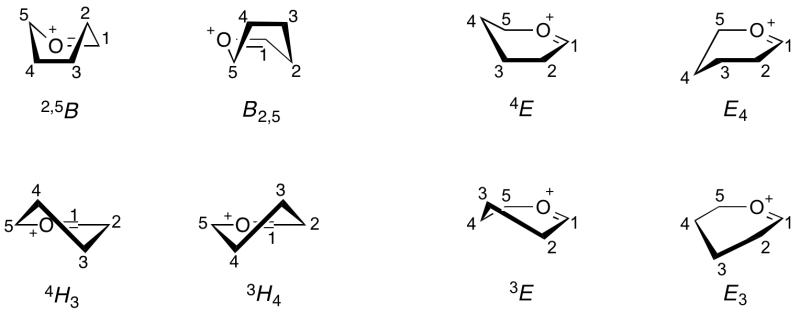
| + | Because the oxocarbenium ion has double-bond character between the anomeric carbon and the ring oxygen, this part of the structure must be planar <cite>Sinnott1990</cite>. Thus for a 6-membered ring, C5, O5, C1 and C2 must lie in a plane. This arrangement can be accommodated in a 6-membered ring in the <sup>4</sup>''H''<sub>3</sub> or <sup>3</sup>''H''<sub>4</sub> half chair conformations; <sup>4</sup>''E'', ''E''<sub>4</sub>, <sup>3</sup>''E'', or ''E''<sub>3</sub> envelope conformations; or <sup>2,5</sup>''B'' or ''B''<sub>2,5</sub> boat conformations. | ||
| + | |||
| + | [[Image:Oxocarbenium_conformations.png|center|800px]] | ||
| + | |||
| + | The complete conformational pseudorotational itinerary for a pyranosyloxocarbenium ion is the same as that for cyclohexene, but with different descriptors: | ||
| + | |||
| + | ... <-> <sup>3</sup>''E'' <-> <sup>3</sup>''H''<sub>4</sub> <-> ''E''<sub>4</sub> <-> <sup>2,5</sup>''B'' <-> ''E''<sub>3</sub> <-> <sup>4</sup>''H''<sub>3</sub> <-> <sup>4</sup>''E'' <-> ''B''<sub>2,5</sub> <-> <sup>3</sup>''E'' <-> ... | ||
==See also== | ==See also== | ||
| Line 17: | Line 27: | ||
==References== | ==References== | ||
<biblio> | <biblio> | ||
| − | # | + | #Banait1991 Banait, N. S., and Jencks, W. P. (1991) ''Journal of the American Chemical Society'' 113, 7951-7958. |
| + | #Sinnott1990 Sinnott, M.L., (1990) ''Chemical Reviews'', 90, 1171-1202. | ||
</biblio> | </biblio> | ||
[[Category:Definitions and explanations]] | [[Category:Definitions and explanations]] | ||
Latest revision as of 03:32, 24 March 2017
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
- Author: Stephen Withers
- Responsible Curator: Spencer Williams
An oxocarbenium ion is the species that is formed upon formal ‘ionization’ of a glycosidic bond. Also called an oxacarbenium ion, or glycosylium ion, it is a positively charged carbocation that is stabilized by resonance.
Lifetime and involvement in enzymatic mechanisms
Lifetimes of oxocarbenium ions in water are estimated at around 10-12 sec: right at the limit of existence as free species. Further, in the presence of anionic nucleophiles their lifetime was shown to be considerably shorter [1]. Therefore it seems unlikely that oxocarbenium ion intermediates exist in the active sites of glycoside hydrolases, which contain nucleophilic carboxylate residues. Indeed there is no experimental evidence for their existence. However, kinetic isotope effect studies have shown that transition states for glycosyl transfer have considerable oxocarbenium ion character.
For glycosyltransferases the situation is less clear, and there remains the possibility that an oxocarbenium ion may represent a bonafide reaction intermediate. For this to be the case the active site must effectively exclude water and must not contain nucleophilic residues in close proximity to the reactive centre.
Conformation
Because the oxocarbenium ion has double-bond character between the anomeric carbon and the ring oxygen, this part of the structure must be planar [2]. Thus for a 6-membered ring, C5, O5, C1 and C2 must lie in a plane. This arrangement can be accommodated in a 6-membered ring in the 4H3 or 3H4 half chair conformations; 4E, E4, 3E, or E3 envelope conformations; or 2,5B or B2,5 boat conformations.
The complete conformational pseudorotational itinerary for a pyranosyloxocarbenium ion is the same as that for cyclohexene, but with different descriptors:
... <-> 3E <-> 3H4 <-> E4 <-> 2,5B <-> E3 <-> 4H3 <-> 4E <-> B2,5 <-> 3E <-> ...
See also
References
-
Banait, N. S., and Jencks, W. P. (1991) Journal of the American Chemical Society 113, 7951-7958.
-
Sinnott, M.L., (1990) Chemical Reviews, 90, 1171-1202.