CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Difference between revisions of "Glycoside Hydrolase Family 73"
| (84 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | <!-- RESPONSIBLE CURATORS: Please replace the {{UnderConstruction}} tag below with {{CuratorApproved}} when the page is ready for wider public consumption --> | ||
| − | {{ | + | {{CuratorApproved}} |
| − | * [[Author]]: | + | * [[Author]]: [[User:Florence Vincent|Florence Vincent]] |
| − | * [[Responsible Curator]]: | + | * [[Responsible Curator]]: [[User:Bernard Henrissat|Bernard Henrissat]] |
---- | ---- | ||
| Line 15: | Line 15: | ||
|- | |- | ||
|'''Mechanism''' | |'''Mechanism''' | ||
| − | | | + | |Inverting and Neighboring group participation |
|- | |- | ||
|'''Active site residues''' | |'''Active site residues''' | ||
| Line 28: | Line 28: | ||
== Substrate specificities == | == Substrate specificities == | ||
| − | + | [[Image:Diapositive1recadree.jpg|thumb|500px|right|'''Figure 1.''' Examples of modular GH73 enzymes: SLH: S‐layer homology domains; [[CBM50]]: [[Carbohydrate Binding Module Family 50]]; purple: signal peptide; grey and red: unknown repeated domains. GenBank accession | |
| − | The | + | numbers are indicated for each protein.]] |
| − | + | Family GH73 contains bacterial and viral [[glycoside hydrolase]]s. Most of the enzymes of this family cleave the β-1,4-glycosidic linkage between ''N''-acetylglucosaminyl (NAG) and ''N''-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycans. Because of their cleavage specificity, they are commonly described as β-''N''-acetylglucosaminidases. The enzymes from family GH73 are mainly involved in daughter cell separation during vegetative growth, and they often hydrolyze the septum after cell division (Acp from ''Clostridium perfringens'' <cite>Camiade2010</cite>, AltA from ''Enterococcus faecalis'' <cite>Eckert2006</cite>). Occasionally GH73 enzymes are used during host-cell invasion such as the virulence-associated peptidoglycan hydrolase Auto from ''Listeria monocytogenes'' <cite>Bublitz2009</cite>. | |
| + | |||
| + | GH73 enzymes are mostly surface-located and often exhibit repeated sequences that could be involved in bacterial cell-wall binding (figure 1). Unknown repeated domains are appended for instance to LytD and LytG from ''Bacillus subtilis'' <cite>Rashid1995 Horsburgh2003</cite>, AcmB from ''Lactococcus lactis'' <cite>Huard2003</cite>, and Auto from ''L. monocytogene'' <cite>Bublitz2009</cite>. Some of these repeated domains have been identified such as the carbohydrate-binding modules of family [[CBM50]] (also known as LysM domains) appended for instance to AcmA of ''Lactococcus lactis'' <cite>Inagaki2009</cite>, AltA from ''Enterococcus faecalis'' <cite>Eckert2006</cite> and Mur2-Mur2 from ''Enterococcus hirae'' <cite>Eckert2007</cite>. | ||
== Kinetics and Mechanism == | == Kinetics and Mechanism == | ||
| − | No kinetic parameters have been determined for any enzyme of the GH73 family, as the production of synthetic peptidoglycan substrates remains a | + | No kinetic parameters have been determined for any enzyme of the GH73 family, as the production of synthetic peptidoglycan substrates remains a challenge. |
| + | |||
| + | == Three-dimensional structures == | ||
| + | [[Image:auto-flgjSURFnew.jpg|thumb|300px|right|'''Figure 2.''' Ribbon diagram of Auto structure (orange) and its surface, superimposed on FlgJ structure (green).]] | ||
| + | Crystal structures of GH73 are available and have been reported simultaneously, namely FlgJ from ''Sphingomonas sp.'' (SPH1045-C) <cite>Hashimoto2009</cite> and Auto a virulence associated peptigoglycan hydrolase from ''Listeria monocytogenes'' <cite>Bublitz2009</cite>. The two GH73 show the same fold, with two subdomains consisting of a β-lobe and an α-lobe that together create an extended substrate binding groove (Figure 2). With a typical lysozyme (α+β) fold, the catalytic domain of Auto is structurally related to the catalytic domain of Slt70 from ''E. coli'' <cite>vanAsselt1999</cite>, the family [[GH19]] chitinases and goose egg-white lysozyme (GEWL, [[GH23]]) <cite>Weaver1995</cite>. FlgJ is structurally related to a peptidoglycan degrading enzyme from the bacteriophage phi 29 <cite>Xiang2008</cite> and also to family [[GH22]] and [[GH23]] lysozymes. | ||
== Catalytic Residues == | == Catalytic Residues == | ||
| + | [[File: FigurePourCazypedia2022.jpg|thumb|300px|right|'''Figure 3.''' The motion performed by the β-hairpin to close on the active site is shown by comparing FlgJSt, Auto, and AtlA β-hairpin’s positions]] The catalytic [[general acid]] is a glutamate, strictly conserved in the GH73 family. Its catalytic role has been evidenced in FlgJ <cite>Maruyama2010</cite>, Auto <cite>Bublitz2009</cite>, AcmA <cite>Inagaki2009</cite> and AltWN <cite>Yokoi2008</cite>. Glu185 in FlgJ and Glu122 in Auto have also been identified through structural comparison with the actives sites of [[GH19]], [[GH22]] and [[GH23]] enzymes <cite>Hashimoto2009 Bublitz2009 </cite>. However, in contrast to [[GH22]] lysozymes, the structures of FlgJ and Auto both lack a nearby second catalytic carboxylate such as Asp52 in hen egg white lysozyme (HEWL), which represents the [[catalytic nucleophile]] <cite>Vocadlo2001</cite>. Interestingly this amino acid is strictly conserved in the sequences of GH73 enzymes but it is situated 13Å away from the Glu [[general acid]] in the active site. Recent work on AtlA from ''Enterococcus faecalis'' identifies key conserved catalytic residues and together with a closed conformation of the active site groove confirms the ([[inverting mechanism]])<cite>Roig-Zamboni2022</cite> (see figure 3). | ||
| − | + | On the other hand, significant residual activity was found when the putative nucleophile/base residue of AcmA and AltWN were converted to glutamine or asparagine (for Asp1275 in AltWN), which is more compatible with substrate-assisted catalysis (also termed "[[neighboring group participation]]" mechanism) involving anchimeric assistance by the acetamido group of the GlcNAc moiety. In such a mechanism, a neighboring tyrosine is frequently involved. In family GH73, a Tyr residue is highly conserved (Fig2: Tyr220 in Auto), in close proximity to the catalytic [[general acid]] Glu. Substitution of this Tyr residue in FlgJ, AcmA and AltWN was associated with a reduced activity similar to that resulting from the mutation of the [[general acid]] Glu <cite>Maruyama2010 Inagaki2009 Yokoi2008</cite>. The [[neighboring group participation]] mechanism involving the [[general acid]] Glu and the Tyr as essential catalytic residues found support from the sequence comparison of family GH73 with families [[GH20]], [[GH18]], [[GH23]] and [[GH56]], which do not have a catalytic nucleophile residue <cite>Inagaki2009</cite>. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
== Family Firsts == | == Family Firsts == | ||
| − | ;First | + | ;First [[general acid/base]]/[[general acid]] residue identification: Glu 1238 in AltWN from ''Staphylococcus warneri'' M <cite>Yokoi2008</cite> |
| − | + | ;First 3-D structure: peptidoglycan hydrolase FlgJ from ''Sphingomonas sp.'' <cite>Hashimoto2009</cite> | |
| − | |||
| − | ;First 3-D structure: | ||
== References == | == References == | ||
| Line 76: | Line 66: | ||
#Weaver1995 pmid=7823320 | #Weaver1995 pmid=7823320 | ||
#Xiang2008 pmid=18606992 | #Xiang2008 pmid=18606992 | ||
| + | #Vocadlo2001 pmid=11518970 | ||
| + | |||
| + | #Roig-Zamboni2022 pmid=35398351 | ||
</biblio> | </biblio> | ||
[[Category:Glycoside Hydrolase Families|GH073]] | [[Category:Glycoside Hydrolase Families|GH073]] | ||
Latest revision as of 03:48, 27 December 2022
This page has been approved by the Responsible Curator as essentially complete. CAZypedia is a living document, so further improvement of this page is still possible. If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail.
| Glycoside Hydrolase Family GH73 | |
| Clan | none, α+β "lysozyme fold" |
| Mechanism | Inverting and Neighboring group participation |
| Active site residues | partially known |
| CAZy DB link | |
| https://www.cazy.org/GH73.html | |
Substrate specificities
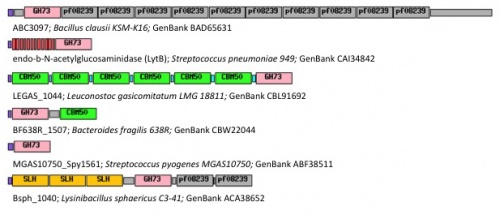
Family GH73 contains bacterial and viral glycoside hydrolases. Most of the enzymes of this family cleave the β-1,4-glycosidic linkage between N-acetylglucosaminyl (NAG) and N-acetylmuramyl (NAM) moieties in the carbohydrate backbone of bacterial peptidoglycans. Because of their cleavage specificity, they are commonly described as β-N-acetylglucosaminidases. The enzymes from family GH73 are mainly involved in daughter cell separation during vegetative growth, and they often hydrolyze the septum after cell division (Acp from Clostridium perfringens [1], AltA from Enterococcus faecalis [2]). Occasionally GH73 enzymes are used during host-cell invasion such as the virulence-associated peptidoglycan hydrolase Auto from Listeria monocytogenes [3].
GH73 enzymes are mostly surface-located and often exhibit repeated sequences that could be involved in bacterial cell-wall binding (figure 1). Unknown repeated domains are appended for instance to LytD and LytG from Bacillus subtilis [4, 5], AcmB from Lactococcus lactis [6], and Auto from L. monocytogene [3]. Some of these repeated domains have been identified such as the carbohydrate-binding modules of family CBM50 (also known as LysM domains) appended for instance to AcmA of Lactococcus lactis [7], AltA from Enterococcus faecalis [2] and Mur2-Mur2 from Enterococcus hirae [8].
Kinetics and Mechanism
No kinetic parameters have been determined for any enzyme of the GH73 family, as the production of synthetic peptidoglycan substrates remains a challenge.
Three-dimensional structures
Crystal structures of GH73 are available and have been reported simultaneously, namely FlgJ from Sphingomonas sp. (SPH1045-C) [9] and Auto a virulence associated peptigoglycan hydrolase from Listeria monocytogenes [3]. The two GH73 show the same fold, with two subdomains consisting of a β-lobe and an α-lobe that together create an extended substrate binding groove (Figure 2). With a typical lysozyme (α+β) fold, the catalytic domain of Auto is structurally related to the catalytic domain of Slt70 from E. coli [10], the family GH19 chitinases and goose egg-white lysozyme (GEWL, GH23) [11]. FlgJ is structurally related to a peptidoglycan degrading enzyme from the bacteriophage phi 29 [12] and also to family GH22 and GH23 lysozymes.
Catalytic Residues
The catalytic general acid is a glutamate, strictly conserved in the GH73 family. Its catalytic role has been evidenced in FlgJ [13], Auto [3], AcmA [7] and AltWN [14]. Glu185 in FlgJ and Glu122 in Auto have also been identified through structural comparison with the actives sites of GH19, GH22 and GH23 enzymes [3, 9]. However, in contrast to GH22 lysozymes, the structures of FlgJ and Auto both lack a nearby second catalytic carboxylate such as Asp52 in hen egg white lysozyme (HEWL), which represents the catalytic nucleophile [15]. Interestingly this amino acid is strictly conserved in the sequences of GH73 enzymes but it is situated 13Å away from the Glu general acid in the active site. Recent work on AtlA from Enterococcus faecalis identifies key conserved catalytic residues and together with a closed conformation of the active site groove confirms the (inverting mechanism)[16] (see figure 3).
On the other hand, significant residual activity was found when the putative nucleophile/base residue of AcmA and AltWN were converted to glutamine or asparagine (for Asp1275 in AltWN), which is more compatible with substrate-assisted catalysis (also termed "neighboring group participation" mechanism) involving anchimeric assistance by the acetamido group of the GlcNAc moiety. In such a mechanism, a neighboring tyrosine is frequently involved. In family GH73, a Tyr residue is highly conserved (Fig2: Tyr220 in Auto), in close proximity to the catalytic general acid Glu. Substitution of this Tyr residue in FlgJ, AcmA and AltWN was associated with a reduced activity similar to that resulting from the mutation of the general acid Glu [7, 13, 14]. The neighboring group participation mechanism involving the general acid Glu and the Tyr as essential catalytic residues found support from the sequence comparison of family GH73 with families GH20, GH18, GH23 and GH56, which do not have a catalytic nucleophile residue [7].
Family Firsts
- First general acid/base/general acid residue identification
- Glu 1238 in AltWN from Staphylococcus warneri M [14]
- First 3-D structure
- peptidoglycan hydrolase FlgJ from Sphingomonas sp. [9]
References
Error fetching PMID 17041059:
Error fetching PMID 19210622:
Error fetching PMID 7581999:
Error fetching PMID 12525152:
Error fetching PMID 12634338:
Error fetching PMID 19686822:
Error fetching PMID 17258207:
Error fetching PMID 18440165:
Error fetching PMID 19351587:
Error fetching PMID 20586063:
Error fetching PMID 10452894:
Error fetching PMID 7823320:
Error fetching PMID 18606992:
Error fetching PMID 35398351:
- Error fetching PMID 20190047:
- Error fetching PMID 17041059:
- Error fetching PMID 19210622:
- Error fetching PMID 7581999:
- Error fetching PMID 12525152:
- Error fetching PMID 12634338:
- Error fetching PMID 19686822:
- Error fetching PMID 17258207:
- Error fetching PMID 19351587:
- Error fetching PMID 10452894:
- Error fetching PMID 7823320:
- Error fetching PMID 18606992:
- Error fetching PMID 20586063:
- Error fetching PMID 18440165:
- Vocadlo DJ, Davies GJ, Laine R, and Withers SG. (2001). Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature. 2001;412(6849):835-8. DOI:10.1038/35090602 |
- Error fetching PMID 35398351:

