CAZypedia celebrates the life of Senior Curator Emeritus Harry Gilbert, a true giant in the field, who passed away in September 2025.
CAZypedia needs your help!
We have many unassigned pages in need of Authors and Responsible Curators. See a page that's out-of-date and just needs a touch-up? - You are also welcome to become a CAZypedian. Here's how.
Scientists at all career stages, including students, are welcome to contribute.
Learn more about CAZypedia's misson here and in this article. Totally new to the CAZy classification? Read this first.
Carbohydrate Binding Module Family 103
This page is currently under construction. This means that the Responsible Curator has deemed that the page's content is not quite up to CAZypedia's standards for full public consumption. All information should be considered to be under revision and may be subject to major changes.
| CAZy DB link | |
| https://www.cazy.org/CBM103.html |
Ligand specificities
The CBM103-containing surface glycan-binding protein (SGBP) of Bacteroides fluxus (BfSGBP-B), a strain from the human intestinal tract, binds laminarin (Laminaria digitata) and mixed-linkage β(1,3)/β(1,4)-glucans (MLG, from Barley) as well as oligosaccharides thereof [1, 2]. The protein binds laminarin and MLG with similar affinity (Ka 8.63 X 104 M-1 and Ka 8.56 X 104 M-1, respectively) [1]. Binding of laminarin-derived oligosaccharides required a degree of polymerization (DP) of more than two and affinities ranged from Kd 3.63-7.61 x 10-5 M (DP3-6) [2]. DP3 and DP4 derived from MLG were binding with similar affinities (Kd 1.23 x 10-5 M or 1.16 x 10-5 M, respectively), but a β(1,3)-glucosyl linkage at the reducing end was essential for binding.
Similarly, a dissected CBM103 of a multimodular laminarinase (GH16_3) of the marine flavobacterium Christiangramia forsetii KT0803T was binding laminarin (Laminaria digitata) and MLG (from Icelandic moss, lichenan) as confirmed by affinity gel electrophoresis [3].
Structural Features
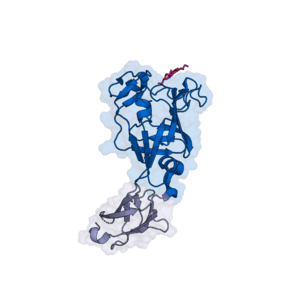
The CBM103 of the B. fluxus SGBP (BfSGBP-B) displays a β-barrel fold with an additional β-strand pair and two short α-helices [2] (Figure 1). 3D crystal structures showed that reducing ends of laminarin- or MLG-derived oligosaccharides bind on the top face of the β-barrel, which provides a 'shallow binding canyon'. Therefore, CBM103 classifies as a type C CBM. Polar- and CH-π-interactions mediate binding to trioses derived from laminarin and MLG, with Trp164 (third subsite), Trp165, Lys172 and Asp221 (all terminal subsite) demonstrated to be indispensable [2]. In the B. fluxus SGBP, the CBM103 follows an N-terminal ‘PKD’ domain.
Functionalities
The CBM103-containing SGBP of B. fluxus (BfSGBP-B) was suggested to support glycan recognition and its recruiting to the cellular surface in the human intestinal tract [2]. Similarly, in 555 representative bacterial metagenome assembled genomes retrieved from phytoplankton blooms of three respective years (2016, 2018 and 2020) in the North Sea, 43 CBM103-only sequences were identified, some of which might function as SGBPs [3]. All detected CBM103-containing sequences (82) belonged to the phylum Bacteroidota and covered also 17 CBM103-CBM102 combinations, 21 CBM103-GH16_3 combinations as well as one CBM103-CBM6-GH5_46 combination. In C. forsetii, the CBM103 is attached to a GH16_3, which was suggested to increase catalysis [3].
Family Firsts
- First Identified
CBM103 was first identified in an SGBP of B. fluxus (BfSGBP-B) [2], although binding of the SGBP to β-glucans was shown before [1]. Later, a CBM103-containing GH16_3 of C. forsetii led to the creation of the CBM family 103 [3].
- First Structural Characterization
The CBM103-containing SGBP of B. fluxus represents the first 3D crystal structure, with and without ligands (PDB ID 7KV5 without ligand, PDB ID 7KV6 with DP3 from MLG, PDB ID 7KV7 with DP3 from laminarin) [2].
References
- Déjean G, Tamura K, Cabrera A, Jain N, Pudlo NA, Pereira G, Viborg AH, Van Petegem F, Martens EC, and Brumer H. (2020). Synergy between Cell Surface Glycosidases and Glycan-Binding Proteins Dictates the Utilization of Specific Beta(1,3)-Glucans by Human Gut Bacteroides. mBio. 2020;11(2). DOI:10.1128/mBio.00095-20 |
- Tamura K, Dejean G, Van Petegem F, and Brumer H. (2021). Distinct protein architectures mediate species-specific beta-glucan binding and metabolism in the human gut microbiota. J Biol Chem. 2021;296:100415. DOI:10.1016/j.jbc.2021.100415 |
- Zühlke MK, Ficko-Blean E, Bartosik D, Terrapon N, Jeudy A, Jam M, Wang F, Welsch N, Dürwald A, Martin LT, Larocque R, Jouanneau D, Eisenack T, Thomas F, Trautwein-Schult A, Teeling H, Becher D, Schweder T, and Czjzek M. (2024). Unveiling the role of novel carbohydrate-binding modules in laminarin interaction of multimodular proteins from marine Bacteroidota during phytoplankton blooms. Environ Microbiol. 2024;26(5):e16624. DOI:10.1111/1462-2920.16624 |